Figure 3.
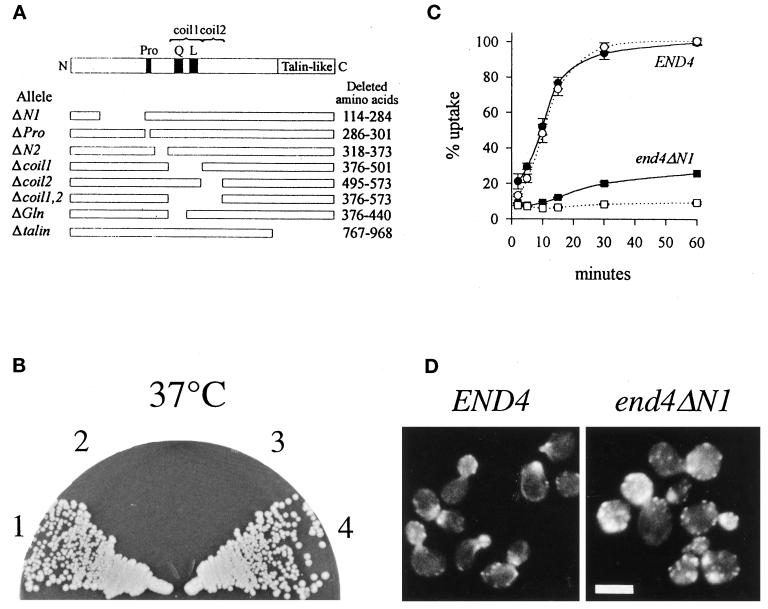
N terminus of End4p is essential for actin organization and endocytosis. (A) Mutant end4 alleles devoid of the indicated regions (open space in the schematic representation) were generated as described in MATERIALS AND METHODS. Pro, proline-rich sequence; Q, glutamine-rich sequence; L, putative leucine-zipper; coil1 and coil2, putative coiled-coil regions 1 and 2, respectively. Proline at aa 498 divides the region from aa 376 to aa 573 into two separate coiled-coil domains (MATERIALS AND METHODS). The nomenclature of end4 alleles and the deleted amino acids are indicated. (B) END4 (section 1, RH3393), end4Δ (section 2, RH3212), end4ΔN1 (section 3, RH3769), and end4Δcoil1 (section 4, RH3395) strains were streaked onto YPUADT plates and incubated for 2 d at 37°C. Deletion of the N-terminal part of End4p but not deletion of the coiled-coil domain impairs growth at higher temperatures. (C) Receptor-mediated endocytosis of radiolabeled pheromone in END4 (RH3393) and end4ΔN1 (RH3769) strains after a 5-min preincubation at either 24°C (solid symbols) or 37°C (open symbols). (D) END4 (RH3393) and end4ΔN1 (RH3769) strains were grown at 24°C and processed for visualization of filamentous actin with rhodamine-conjugated phalloidin as described in MATERIALS AND METHODS. Bar, 5 μm.