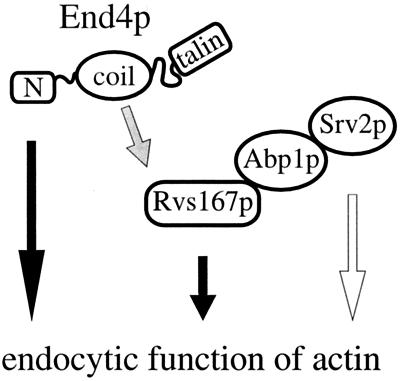
Figure 6.
Hypothetical model. Two parts of End4p participate in endocytosis: the N terminus, which is essential for endocytosis and actin organization, and the coiled-coil domain, which mediates an endocytic function redundant with Abp1p and Srv2p. We propose that this function involves binding to Rvs167p, a protein itself required for endocytosis. The shaded arrow indicates that the physical interaction of End4p and Rvs167p is based solely on two-hybrid data. The open arrow indicates that a recessive negative mutation but not a deletion of SRV2 results in a defect in endocytosis (see text for further explanations).