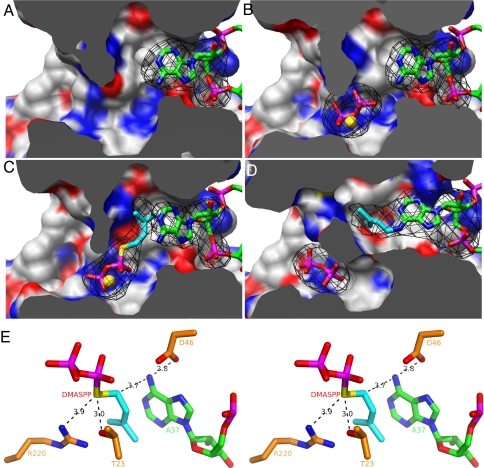
Fig. 5.
Snapshots of the DMATase-catalyzed reaction. (A) Structure of DMATase–tRNA binary complex. DMATase is depicted as protein surface, with carbon in white, nitrogen in blue, and oxygen in red. tRNA is depicted and colored as in Fig. 3. The structure of DMATase is clipped across the reaction channel to reveal contents in the channel. (B) Structure of DMATase–tRNA–PP ternary complex. The pyrophosphate is in sticks, and Mg2+ is depicted as a sphere in gold. (C) Structure of DMATase–tRNA–DMASPP ternary complex. The bridging sulfur and the DMA moiety in DMASPP are colored yellow and cyan, respectively. (D) Structure of DMATase–tRNA(P)–PP ternary complex. A37 of the tRNA substrate in A–C, i6A37 of the tRNA product in D, and pyrophosphate, DMASPP, and Mg2+ in A–D are covered with simulated annealing omit maps colored black. The maps are contoured at 5.0 σ. (E) Stereo view of the final model of DMATase–tRNA–DMASPP ternary complex near the active site showing the catalytic apparatus and substrates. The distances between atoms relevant to mechanistic discussions are labeled in black.