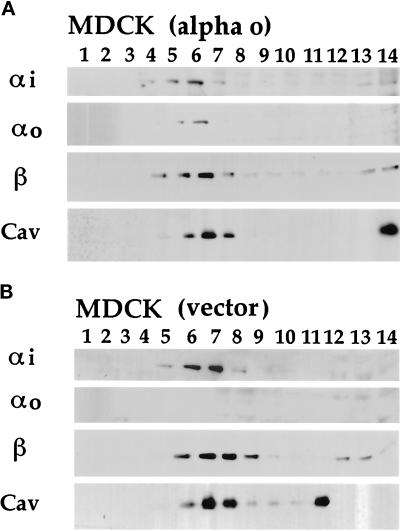
Figure 4.
Fractionation of endogenous and ectopically expressed G proteins and caveolin from TX-100 extracts of MDCK epithelial cells. Stably transfected MDCK cells that ectopically express αo (panel A) were compared with G418-resistant control cells that had been stably transfected with the empty vector (panel B). Four milliliters of detergent extract, adjusted to 40% sucrose, was loaded at the bottom of a tube followed by a 7-ml linear gradient of 30–5% sucrose. After centrifugation, 0.8-ml fractions were collected from the top of the gradients, half of which was acetone-precipitated and analyzed by Western immunoblotting. Most of the cellular protein (assayed by Ponceau S staining of the blots, not shown) remains below the gradient in the five bottom fractions (numbered 10–14). Ectopically expressed αo (A) consistently comigrates with endogenous αi, β, and caveolin (cav) in detergent-resistant, low-density fractions centered around fraction 6 (A and B). αi was detected by B087 antiserum (1:10,000 dilution), β by B600 antiserum (1:10,000), αo by culture medium from Mab 2A-producing cells (1:500), and caveolin (cav) by the purified polyclonal antibodies (30 ng/ml). Not shown are results for αs, which comigrates with the other G protein subunits from MDCK and MA104 cells.