Figure 1. vGluT1 antibody specificity.
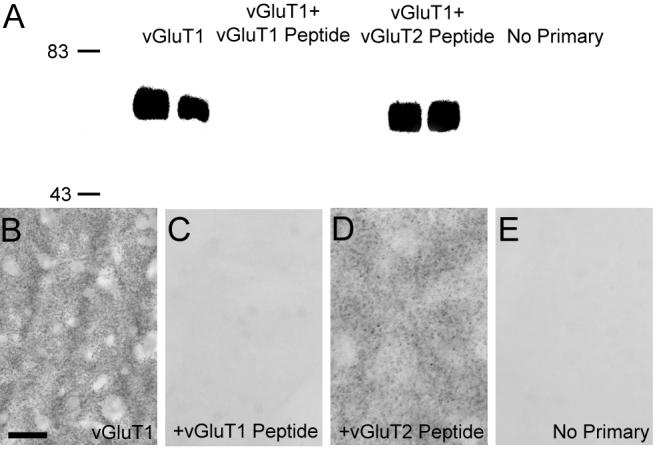
(A) Western blot analysis demonstrating the specificity of antibodies against vGluT1. Anti-vGluT1 antibodies detected proteins of ≈60 kDa corresponding to the molecular weight predicted for the vGluT1 protein in striatal tissue from two different rats. The immunoreactivity is completely abolished when antibodies are omitted (no primary) or preadsorbed with the synthetic vGluT1 peptide prior to immunoblotting, but it is preserved when anti-vGluT1 antibody is preadsorbed with synthetic vGluT2 peptide. (B-E) Light microscopic examination of rat striatal tissue stained with anti-vGluT1 antibodies demonstrates neuropil immunoreactivity (B), which is abolished when antibodies are preadsorbed with the synthetic peptide (C). Immunoreactivity is preserved when antibodies are preadsorbed with the vGluT2 peptide (D). Immunostaining of striatal tissue without primary antibodies is shown for reference (E). Molecular weight standards are indicated on the left (in kDa). Scale bar in B represents 50 μm for B-H.
