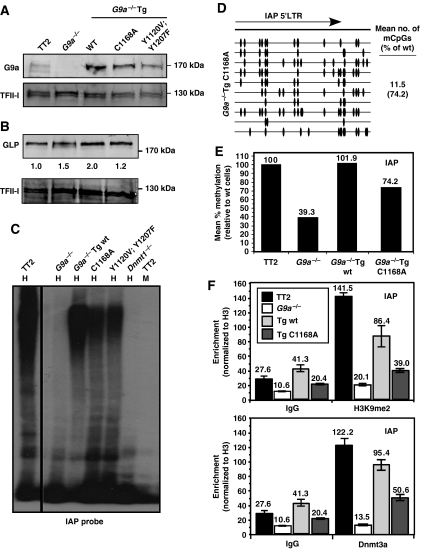
Figure 8.
Stable expression of catalytically inactive G9a transgenes is sufficient to rescue the DNA methylation defect observed in G9a−/− ES cells. The G9a−/− line 2-3 was stably transfected with constructs encoding a wt G9a transgene G9a−/−Tg(wt), or the mutant G9a transgenes G9a−/−Tg(C1168A) and G9a−/−Tg(Y1120V;Y1207F). Each of the latter transgenes harbour amino-acid substitutions in the SET domain that reduce the catalytic activity of the encoded protein to <1% of that of the wt protein. (A) Western blot analysis of cell lines expressing each of these transgenes reveals that the mutant proteins are expressed at the expected molecular weight. An antibody specific for TFII-I was used as a loading control. (B) Quantitative western blot analysis of GLP expression in these lines was determined by normalizing to the signal obtained for endogenous TFII-I on the same blot. (C) Genomic DNA isolated from wt (TT2), G9a−/− (2-3), G9a−/−Tg(wt), G9a−/−Tg(C1168A), G9a−/−Tg(Y1120V;Y1207F) and Dnmt1−/− ES cells was digested with HpaII (H) and subject to Southern blotting using an IAP-specific probe. MspI (M) was used as a control. (D) Bisulphite analysis using primers specific for the IAP 5′LTR confirms that expression of catalytically inactive G9a partially rescues the DNA methylation defect. (E) A bar graph showing the mean no. of mCpGs per molecule sequenced is shown for the bisulphite data presented in (D) and Figure 1D. (F) ChIP was conducted on the wt (TT2), G9a−/− (2-3), G9a−/−Tg(wt) and G9a−/−Tg(C1168A) lines using antisera raised against H3K9me2, Dnmt3a or unmodified H3. Nonspecific IgG was included as a control. Real-time PCR of reverse-crosslinked material using IAP-specific primers was carried out in triplicate and enrichment (±s.d.) is presented as the mean percentage of input material immunoprecipitated, normalized to unmodified H3.