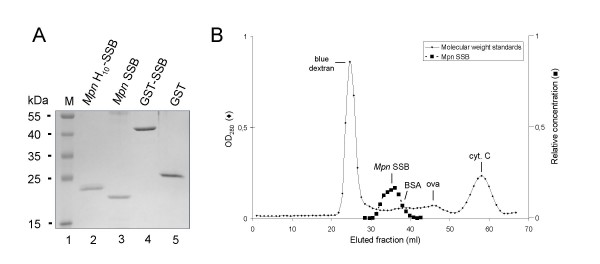
Figure 2.
Purification of recombinant Mpn SSB proteins. (A) Samples of purified recombinant Mpn SSB proteins, i.e. Mpn H10-SSB (lane 2), Mpn SSB (lane 3), GST-SSB (lane 4), and purified GST (lane 5), were analyzed by SDS-PAGE (12%) and Coomassie brilliant blue (CBB)-staining. The sizes of protein markers (lane 1; PageRuler™ Prestained Protein Ladder [Fermentas]) are shown on the left-hand side of the gel. (B) Gel filtration analysis of Mpn SSB. Gel filtration chromatography was performed by applying Mpn SSB to a Sephadex G-150 column. The column was calibrated with blue dextran (2,000 kDa), bovine serum albumin (BSA, 66.4 kDa), ovalbumin (ova, 42.9 kDa), and cytochrome C (cyt. C, 12.3 kDa). Fractions of 1.0 ml were collected and monitored by measuring the optical density at 280 nm (OD280, Y-axis at the left-hand side of the graph). The fractions eluted from the subsequent run containing Mpn SSB were precipitated with trichloroacetic acid, and separated on 14% SDS-PAGE gels. Gels were stained with CBB and recorded using the GelDoc XR system (Bio-Rad). In fractions that contained Mpn SSB, the amount of protein was determined semi-quantitatively using BioNumerics Version 3.0 software (Applied Maths). The relative concentration of Mpn SSB (Y-axis on the right-hand side, in arbitrary units) in these fractions is plotted. In all other fractions, Mpn SSB was not detected by SDS-PAGE analysis and Coomassie brilliant blue-staining.