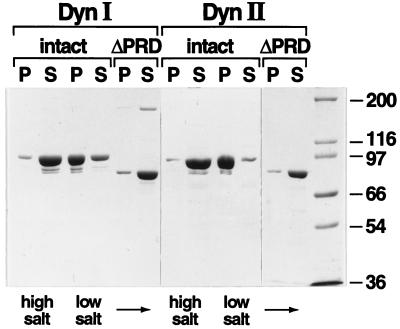
Figure 2.
Self-assembly of dynI and dynII is dependent on an intact PRD. Self-assembly of dynI and dynII and their ΔPRD counterparts was followed by a sedimentation assay that separates dynamin structures that pellet (P) from unassembled dynamin that remains in the supernatant (S) after centrifugation at 100,000 × g for 15 min. Dynamins (in HCB150) were diluted 10-fold to a final protein concentration of 0.2 mg/ml, into either HCB150 (high-salt) or PH (low-salt) buffer. Equal aliquots of soluble and pelleted dynamin were analyzed by SDS-PAGE on 7.5% acrylamide gels and detected by Coomassie brilliant blue staining. The full gels are shown to demonstrate the purity of the protein preparation. Molecular weight protein standards are as indicated.