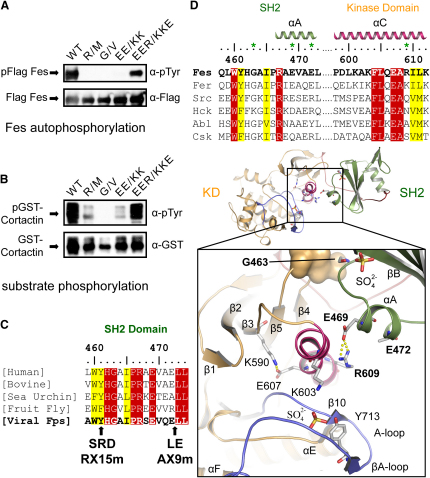
Figure 2.
Activation of Fes Kinase Activity by SH2 Domain Interactions
(A) The effect of mutations in the SH2 domain on Fes kinase activity: HEK293T cells were cotransfected with Flag-tagged Fes and GST-cortactin. Cell lysates were immunoprecipitated with anti-Flag and blotted with anti-pTyr to reveal autophosphorylated (p) Flag-Fes (top panel) or with anti-Flag (lower panel) as a control. WT indicates wild-type Fes, and mutants are described in the text.
(B) GST-cortactin was affinity purified from cells coexpressing WT or mutant Fes proteins and blotted either with anti-pTyr antibodies to measure Fes-induced phosphorylation (p) (top panel) or with anti-GST (bottom panel).
(C) Comparison of the N-terminal SH2 domain sequence of Fps/Fes orthologs. Insertions originally used to identify the SH2 domain in v-Fps are indicated (RX15m and AX9m) (Sadowski et al., 1986; Stone et al., 1984). Conserved residues are highlighted in red and similar residues in yellow.
(D) Details of the Fes SH2-kinase interface. Residues important for the SH2 domain-kinase interaction are conserved in Fps/Fes family members but not in other tyrosine kinases. Residues mutated in this study are indicated by a green asterisk. Interactions of interface residues and the involved secondary structure elements are shown in the structure of active Fes. The alignment of human sequences is colored as in (C).