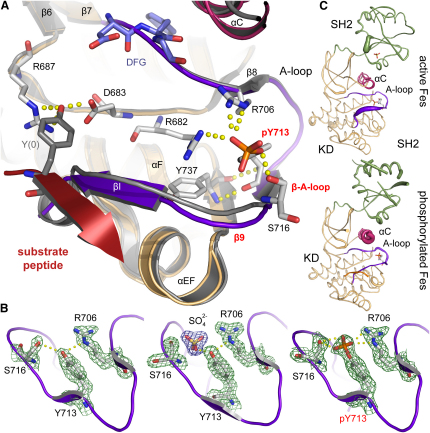
Figure 3.
Conformation of the Fes Activation Segment
(A) Superimposition of the activation segment in active Fes (gray) and the phosphorylated Fes-substrate complex. Hydrophilic interactions formed by the phosphate moiety and the substrate tyrosine are indicated by yellow dots. The antiparallel β sheet formed at the tip of the activation segment is labeled as β-A-loop, and the substrate peptide is shown as a red ribbon. The induced β sheet present in the substrate complex (βI) is also shown.
(B) Interactions of the activation segment Y713 in unphosphorylated (left), active (ligating a sulphate ion; middle), and phosphorylated (right) Fes. A 2Fo – Fc electron density map around the interacting residues is also shown contoured at 2σ.
(C) Schematic drawing of the Fes main chain. Regions with high temperature factors are shown by an increasing radius of the backbone. The activation segment is highlighted in purple; αC, in pink; the SH2 domain, in green; and the substrate peptide (in the phosphorylated Fes structure), in orange. Note the absence of ordered loop regions in the SH2 domain and kinase N lobe of phosphorylated Fes.