Figure 6.
The Abl SH2 Domain Stimulates Kinase Activity
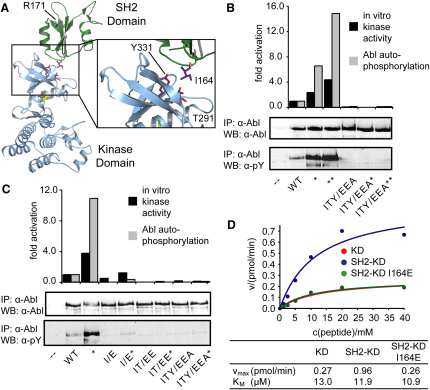
(A) Ribbon representation of the active conformation of Abl (PDB entry 1OPL chain B) and close-up view of SH2-kinase domain interface. Critical interface residues (I164, T291, and Y331) are shown. R171 highlights the location of the pTyr-binding site in the SH2 domain.
(B) SH2-kinase interface residues are required for in vivo Abl activity. HEK293 cells were transfected with c-Abl (WT) or the activated Abl variants PP (∗) or G2A/PP (∗∗) or with the corresponding ITY/EEA mutants, as indicated. Cells were lysed, and anti-Abl immunoprecipitates were analyzed by anti-Abl and anti-pTyr (pY) immunoblotting (lower panels). The histograph shows the in vitro kinase activity (mean of two experiments done in duplicate, black bars) and levels of autophosphorylation (mean of two immunoprecipitations, gray bars) of the Abl constructs relative to c-Abl and corrected for endogenous c-Abl levels.
(C) I164 in the Abl SH2 domain is required for efficient kinase activity (WT = c-Abl, ∗ = Abl-PP; corresponding mutants are indicated).
(D) The SH2 domain is necessary and sufficient to stimulate in vitro Abl kinase activity. Purified Abl proteins (see also Figures S9D and S9E) were evaluated for kinase activity in the presence of 50 μM ATP and the indicated concentrations of an optimal Abl substrate peptide. The Km and vmax values are given below the graph.