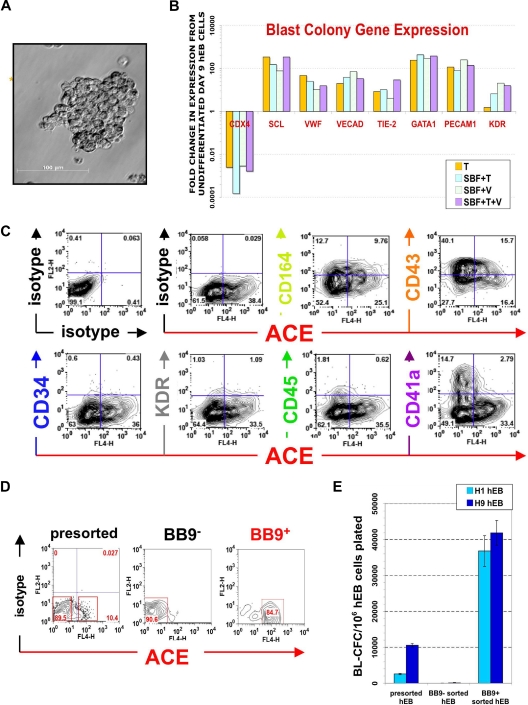
Figure 1.
Phenotype of ACE + blast colonies generated in SF conditions. (A) Typical morphology of a blast colony with loosely packed cells generated from day 7 (line H1) hEB differentiated in BVF2H; 5-6 days post-hEB cell plating. (B) Quantitative RT-PCR expression profiles: day 9 hEB blasts were expanded with 50 ng/mL TPO alone (T), 50 ng/mL each of SCF, BMP4, FGF2/heparin, TPO (SBF + T), 50 ng/mL each of SCF, BMP4, FGF2/heparin, VEGF (SBF + V), or 50 ng/mL each of SCF, BMP4, FGF2/heparin, TPO, and VEGF (SBF + T + V). Blast colonies (∼10-15) from each GF condition were plucked from methylcellulose, pooled for RNA harvest, and analyzed for expression of indicated transcripts by qRT-PCR, using the 2−ΔΔT method25 (see Document S1). Shown are the relative, normalized expressions of CDX4, SCL, VWF, VE-cadherin, TIE-2, GATA1, PECAM1, and KDR/flk1 compared with expressions in control, undifferentiated day 9 hEB cells. qRT-PCR products were verified to be specifically amplified by agarose gel electrophoresis at linear ranges of amplification for the indicated conditions (data not shown). (C) FACS analysis of pooled (∼8-10) blast colonies from day 7 hEB cells revealed abundant expression of ACE/BB9, CD164, CD41a, and CD43, and minor amounts of CD34, KDR, and CD45. BL-CFC activity is contained entirely within BB9+ hEB cell fractions. (D) Single cell suspensions from a representative sorting experiment: day 8 hEB (presorted hEB; hESC lines H1 and H9) were FACS-purified into BB9+ or BB9− sorted populations as described under “Methods.” (E) Eight to 15 × 104 viable, purified day 8 hEB cells, or 15 × 104 viable, presorted total day 8 hEB cells from lines H1 or H9 (differentiated as described in Document S1) were recultured in duplicate in SF methylcellulose containing 50 ng/mL each of BMP4, FGF2/heparin, TPO, VEGF, and IL-6. Blast colonies generated per viable, sorted hEB cells plated were enumerated after 4 to 5 days.