Abstract
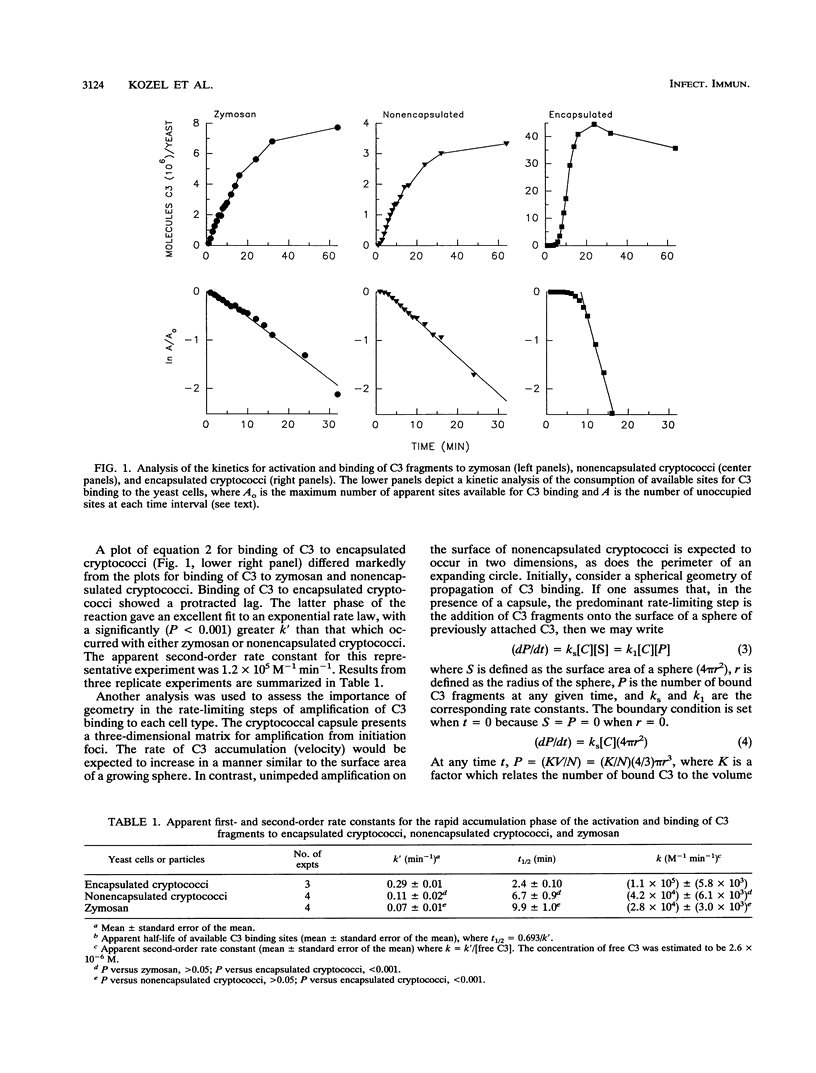
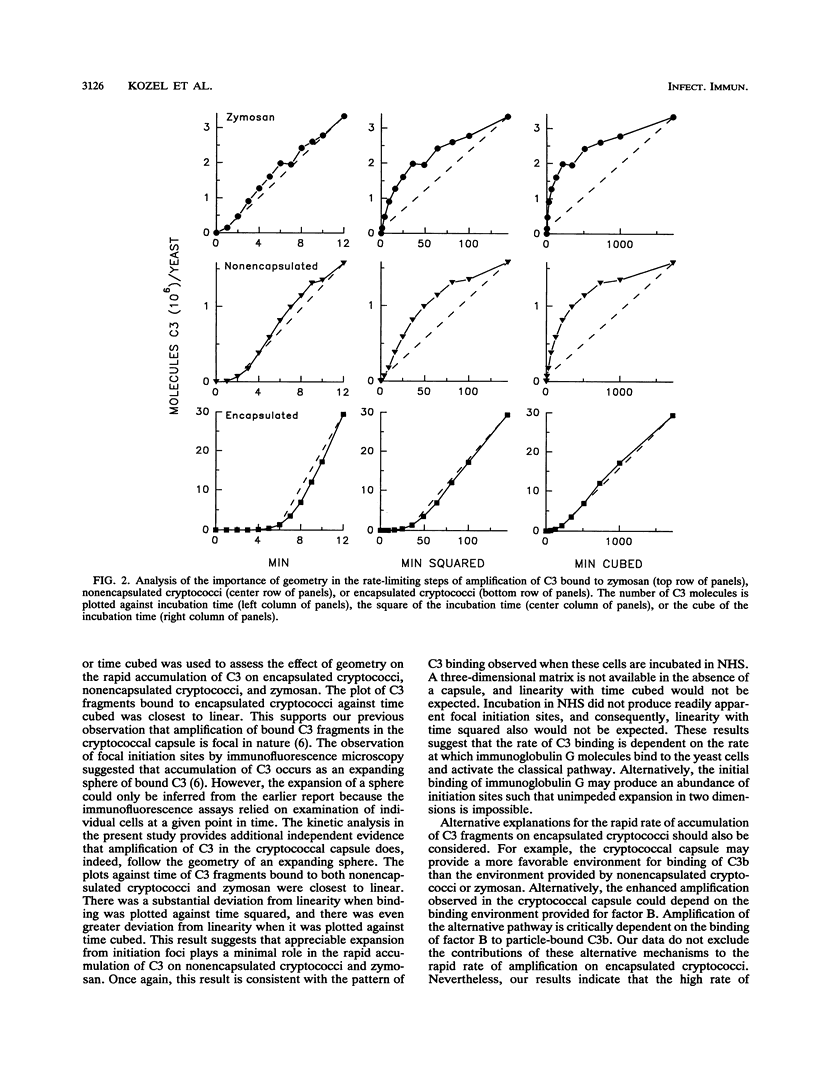
Encapsulated and nonencapsulated cryptococci exhibit quantitative and qualitative differences in their activation of the complement system. We examined the kinetics for the rapid amplification phase in which C3 was activated and bound to encapsulated cryptococci, nonencapsulated cryptococci, and zymosan particles. Yeast cells were incubated in normal human serum containing 125I-labeled C3, and bound C3 fragments were measured after 1 to 64 min of incubation. A kinetic analysis showed that the apparent first-order rate constant (k') for binding of C3 to nonencapsulated cryptococci did not differ significantly from k' for binding of C3 to zymosan particles (P greater than 0.05). However, the rate constant for binding of C3 to encapsulated cryptococci was significantly (P less than 0.001) greater than k' for binding of C3 to nonencapsulated cryptococci and zymosan particles. A plot of C3 molecules bound to encapsulated cryptococci versus time cubed was nearly linear, suggesting that accumulation of C3 in the cryptococcal capsule follows the kinetics predicted by an expanding sphere. In contrast, the plot of C3 molecules bound to nonencapsulated cryptococci or zymosan particles against time was nearly linear, but those plots against time squared or time cubed were not. This result indicates that the rate-limiting step for the addition of C3 fragments to these latter yeast cells follows the kinetics of neither the perimeter of an expanding circle nor the surface of an expanding sphere. Taken together, the results indicate that the high rate of accumulation of C3 in the cryptococcal capsule is consistent with the expected geometry of an expanding sphere of bound C3 within the three-dimensional matrix of the capsule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988 Nov;56(11):2794–2800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Murphy J. W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991 Sep;59(9):3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Pfrommer G. S., Schlageter A. M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989 Jul;57(7):1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. C3b deposition during activation of the alternative complement pathway and the effect of deposition on the activating surface. J Immunol. 1983 Oct;131(4):1930–1935. [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Wilson M. A., Kozel T. R. Contribution of antibody in normal human serum to early deposition of C3 onto encapsulated and nonencapsulated Cryptococcus neoformans. Infect Immun. 1992 Mar;60(3):754–761. doi: 10.1128/iai.60.3.754-761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


