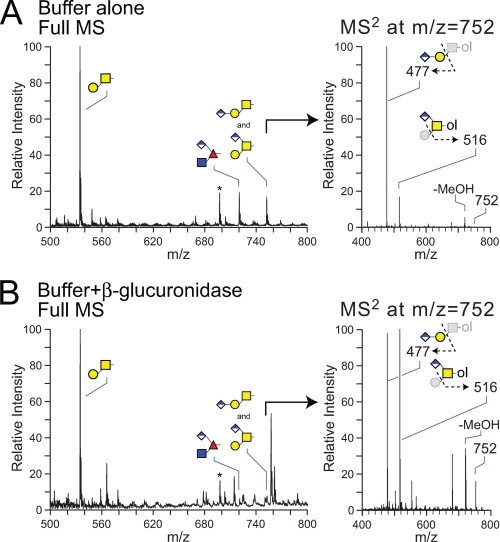
FIGURE 5.
The major hexuronylated O-linked glycans of the Drosophila embryo are sensitive to digestion with β-glucuronidase from H. pomatia. O-Glycans released from fly embryo powder by β-elimination were treated with buffer alone (A) or with β-glucuronidase from H. pomatia (B) before permethylation and analysis by NSI-MS. The core 1 disaccharide (m/z = 534) predominates the MS profile with or without enzymatic digestion. Incubation with the enzyme reduces detected parent ion signals in full MS for both the O-Fuc trisaccharide at m/z = 722 and the core 1 trisaccharide isomers at m/z = 752. A, right panel, without enzyme digestion, the MS/MS spectra for the permethylated HexA1Hex1HexNAc-ol at m/z = 752 exhibits an intense signal at m/z = 477, corresponding to the loss of monosubstituted HexNAc-ol (linear structure) and a characteristic ion at m/z = 516, corresponding to the loss of terminal Hex (branched structure). B, right panel, incubation with β-glucuronidase attenuates the intensity of the MS/MS ion at m/z = 477 (linear trisaccharide) to near equivalence with the m/z = 516 ion (branched trisaccharide). Comparison of the relative signal intensities for the MS/MS ions at m/z = 516 and 752 in panels A and B indicates that the branched core 1 trisaccharide is also reduced by enzyme digestion, although to a lesser extent than the linear trisaccharide (>80% versus ∼30%), indicating partial resistance of the branched structure.