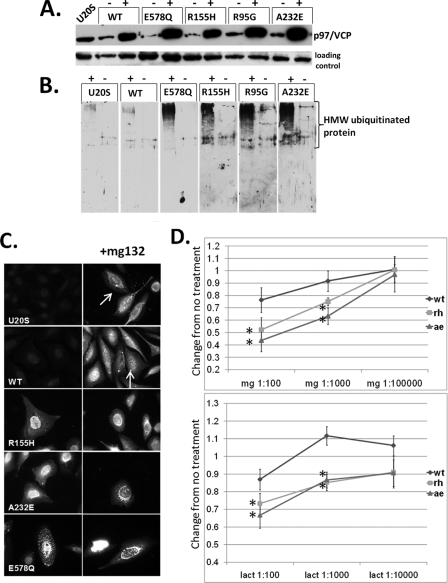
FIGURE 1.
IBMPFD mutant-expressing cells increase ubiquitinated proteins. A, characterization of tetracycline-inducible U20S cell lines expressing Myc-tagged p97/VCP, ATPase inactive p97/VCP-E578Qm, or IBMPFD mutant p97/VCP-R155H, R95G, or A232E. Cell lysates from uninduced (-) and 16 h tetracycline-induced (+) U20S cells were separated by SDS-PAGE and subjected to Western blot analysis to detect Myc-tagged p97/VCP (top panel) or actin (bottom panel). B, the same induced cells as above were immunoblotted with an antibody to ubiquitin. The lanes were taken from the same gel at the same exposure and realigned for presentation purposes. Note the increase in high molecular weight ubiquitinated species in IBMPFD mutant-expressing cells. C, ubiquitin immunostaining of control U20S cells or U20S cells expressing p97/VCP-WT, ATPase inactive p97/VCP-E578Q, or IBMPFD mutant p97/VCP-R155H or -A232E either untreated (left panels) or treated with proteasome inhibitor MG132 (right panels). Note that p97/VCP-WT-expressing cells or untransfected cells have small perinuclear aggresomes that are not present in IBMPFD mutant-expressing cells. D, MTT assays of U20S cells expressing p97/VCP-WT or IBMPFD mutant p97/VCP-R155H or -A232E following 16 h of application of 10-fold dilutions (original concentrations, 1 mm) as indicated of the proteasome inhibitors MG132 (mg, top panel) or lactacystin (lact, bottom panel). Units are the change of treated cells compared with untreated cells (arbitrarily set to 1) in each condition.