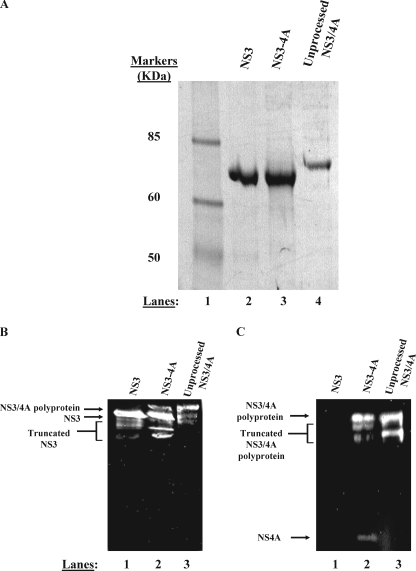
FIGURE 2.
NS3-4A purifies as two separable proteins. A, purified proteins were subjected to denaturing electrophoresis on a 4–12% gradient gel. Purified NS3 (lane 2), NS3-4A (lane 3), and NS3/4A polyprotein (lane 4) were subjected to electrophoresis side by side for comparison of mobilities. The band shown in lane 3 represents the NS3 component of a native, fully cleaved NS3-4A preparation. The band shown in lane 4 represents an NS3/4A polyprotein preparation produced by mutating the Thr/Ser cleavage site between NS3 and NS4A to a non-cleavable sequence (AA). In panels B and C, anti-NS3 and anti-NS4A Western blot analysis confirm the identity of our purified proteins (see “Experimental Procedures”). Panel B depicts an anti-NS3 Western blot. In panel B, lane 1 contains purified NS3, lane 2 contains purified, full-length NS3-4A, and lane 3 contains purified NS3/4A polyprotein. Truncated forms of NS3 are visible below the full-length protein in each lane in the anti-NS3 blot. These truncated forms of NS3 are likely produced during bacterial expression as well as during the multiday purification performed in the absence of protease inhibitors. These truncated forms could represent either N- or C-terminal truncations of NS3 as the monoclonal anti-NS3 antibody binds to the central region of the helicase domain. Panel C depicts an anti-NS4A Western blot. In panel C, lane 1 contains purified NS3, lane 2 contains purified, full-length NS3-4A, and lane 3 contains purified NS3/4A polyprotein. Truncated forms of the NS3/4A polyprotein are visible below the full-length polyprotein in the anti-NS4A blot. These truncated forms of NS3/4A polyprotein likely represent N-terminal degraded NS3/4A as the monoclonal anti-NS4A antibody binds the final 11 C-terminal residues of NS4A.