Abstract
The genetic environment of the blaOXA-18 gene encoding a peculiar clavulanic acid-inhibitable Ambler class D extended-spectrum β-lactamase was determined from the prototype OXA-18-producing Pseudomonas aeruginosa MUS clinical isolate. An 8.2-kb genomic DNA fragment containing blaOXA-18 was cloned from P. aeruginosa MUS. Although most oxacillinases are located in integrons, blaOXA-18 lacked gene cassette-specific features. It was bracketed by two duplicated sequences containing ISCR19, a novel insertion sequence of the ISCR family of mobile elements; ΔintI1, a truncated integrase gene; and a truncated Δaac6′-Ib gene cassette. It is likely that ISCR19 was at the origin of the blaOXA-18 gene mobilization by a rolling-circle transposition event followed by homologous recombination. Furthermore, analysis of the cloned genomic DNA fragment revealed the presence of the integron-containing blaOXA-20 gene. Concomitantly, three P. aeruginosa clinical isolates, displaying a synergy image as determined by double-disk diffusion tests on cloxacillin-containing plates, were isolated from three patients hospitalized in different wards over a 9-month period at the Saint-Luc University hospital (Brussels, Belgium). These isolates were positive by PCR for blaOXA-18 and blaOXA-20 genes, genetically related to P. aeruginosa MUS as determined by pulsed-field gel electrophoresis, and carried the same blaOXA-18/blaOXA-20-associated genetic structures. This report characterized the genetic elements likely at the origin of blaOXA-18 gene mobilization in P. aeruginosa and suggests the spread of oxacillin-type extended-spectrum β-lactamases in P. aeruginosa at the Saint-Luc University hospital of Brussels, Belgium.
Pseudomonas aeruginosa is a predominantly nosocomial pathogen that is increasingly resistant to antibiotics (18). Whereas P. aeruginosa is naturally susceptible to most expanded-spectrum cephalosporins, resistance to these molecules may result from overexpression of the naturally occurring cephalosporinase and from acquired Ambler class A, B, and D extended-spectrum β-lactamases (ESBLs) (2, 18).
Clavulanic acid-inhibitable Ambler class A ESBLs have been identified in P. aeruginosa and include TEM-, SHV-, CTX-M-, PER-, GES-, and VEB-type and BEL-1 β-lactamases (1, 18, 21, 24, 27). Although the definition of ESBLs is often restricted to class A β-lactamases, several oxacillinases with extended-spectrum activity may be also included, and these have been termed OXA-ESBLs (19, 21, 24). These enzymes predominantly occur in P. aeruginosa and mostly derive from OXA-10 (OXA-11, -14, -16, and -17) or OXA-13, which is a 10-amino-acid derivative of OXA-10 (OXA-19 and -28) or to a lesser extent from OXA-2 (OXA-15 and -32) (19, 21, 24). Others OXA-ESBLs are unrelated to any broad-spectrum OXA enzymes, e.g., OXA-18 and OXA-45 (21, 25, 34). Although most OXA-type enzymes are resistant to β-lactamase inhibitors, OXA-18 and OXA-45 are well inhibited by clavulanic acid (19, 25, 34).
The blaOXA-18 gene was initially reported in France from the P. aeruginosa MUS isolate, along with another oxacillinase gene, blaOXA-20 (22, 25). The blaOXA-18 gene has recently been detected in a clinical epidemic clone of P. aeruginosa in Tunisia (15). Unlike the prototype OXA-18-producing P. aeruginosa MUS strain, the Tunisian isolates were blaOXA-20 negative but positive for either TEM-1 or mostly for SHV-1 β-lactamases. In both studies, the blaOXA-18 gene was chromosomally encoded (15, 25), and the Tunisian isolates were clonally related but different from the prototype OXA-18-producing P. aeruginosa MUS strain, as revealed by pulsed-field gel electrophoresis (PFGE) analysis (15).
ISCR are peculiar mobile elements, since they lack the terminal inverted repeats (IRs) typical of most insertion sequence (IS) elements (35). Instead, their termini are distinctive and have different functions (35). Their transposition mechanism may differ from that of most transposable elements in that it involves a rolling-circle (RC) transposition mechanism (6, 33, 35). The single protein encoded by each element is the cognate transposase, which is responsible for initiating replication at one end of the element (called oriIS) and is also believed to be involved in terminating replication at the other end of the element (called terIS) (35). ISCR have been found to be associated with several resistance genes of different families of antibiotics that are disseminating (35).
In the present study, the genetic environment of the blaOXA-18 gene has been characterized from the prototype strain P. aeruginosa MUS clinical isolate and compared to that of OXA-18 expressing P. aeruginosa strains isolated from patients hospitalized at Saint-Luc University hospital in Brussels, Belgium. Unlike most oxacillinases, blaOXA-18 was not in a form of gene cassette. The blaOXA-18 gene was surrounded by insertion sequences ISCR19 of the ISCR family, likely at the origin of its acquisition. Furthermore, the present study demonstrates the ongoing emergence of this type of resistance determinant now in clinical P. aeruginosa isolates.
MATERIALS AND METHODS
Bacterial strains, plasmids, electroporation, and culture conditions.
The clinical P. aeruginosa isolate MUS produces OXA-18 and OXA-20 β-lactamases (22, 25). P. aeruginosa 1-63, P. aeruginosa 1-52, and P. aeruginosa 1-22 strains were isolated in 2006 and in 2007 from in-patients hospitalized at Saint-Luc University Hospital, Brussels, Belgium (Table 1), and were identified by conventional microbiological methods (API-20NE; bioMérieux, Marcy l'Etoile, France).
TABLE 1.
Primers used in this study
| Primer | No.a | Sequence (5′-3′) | Source or reference |
|---|---|---|---|
| OXA-18B | 1 | TTGGCATCGGAAAGCGAACC | 25 |
| OXA-18F | 2 | ATTTCAACGGTTTGCGACG | 25 |
| 5′CSINV | 3 | GCTCCATAACATCAAACATC | This study |
| T3-666 | 4 | GGGCGCAGATGGTGATGTCG | This study |
| T3.4 | 5 | TCAGCTCGATGAAGGTTTCCA | This study |
| T7.2 | 6 | GGAAACCTTCATCGAGCTGAT | This study |
| MD32.9 | 7 | TCGGTCTCCACGCATCG | This study |
| T3-811 | 8 | CCCCGATGGCGTCAACTGTG | This study |
| 5′CS | 9 | GGCATCCAAGCAGCAAG | 29 |
| AAC6′Ib-B | 10 | CGTTTGGATCTTGGTGACCT | This study |
| STR-A-B | 11 | ATTGATCAACCGATAGGCTG | This study |
| OXA-20B | 12 | AGAATAGCACGCGCAATTGC | 22 |
| OXA-20F | 13 | CTGTTGTACTTGTCTCTCTTGG | 22 |
| Oxa18-GPS1 | 14 | TTCCGGCTTGTAATCCCAG | This study |
| Oxa18-GPS2 | 15 | GTCGATAACAAGCGTGCAGG | This study |
| Oxa18-GPS3 | 16 | CATGGACAGGCTCCGTTGCAT | This study |
| Oxa18-GPS4 | 17 | CACTCGACGATATGAGATCG | This study |
See numbering in Fig. 1.
Electrocompetent Escherichia coli DH10B (Invitrogen, Eragny, France) and P. aeruginosa KG2505, which does not express the naturally and chromosome-encoded AmpC β-lactamase and is deficient for the multidrug efflux pump MexAB-OprM (23) were used as a recipient in electroporation experiments. E. coli J53AzR strain, which is resistant to sodium azide, and ciprofloxacin-resistant P. aeruginosa PU21 (12) were used for conjugation experiments. E. coli 50192 was used as a source of high size plasmid marker (12, 25). The plasmid vector pBKCMV carrying a kanamycin resistance marker was used for cloning experiments (25). Bacterial cells were grown in Trypticase soy (TS) broth and on TS agar plates (Bio-Rad, Marnes-La-Coquette, France).
Antimicrobial agents and susceptibility testing.
Routine antibiograms were determined by the disk diffusion method on Mueller-Hinton (MH) agar (Bio-Rad). The antimicrobial agents and their sources have been described elsewhere (25). MICs of β-lactams were determined and interpreted as described previously (4). The double-disk synergy test was performed with expanded-spectrum cephalosporins and ticarcillin-clavulanic acid disks on MH cloxacillin (250 μg/ml)-containing agar plates (13, 20).
Plasmid content, mating out, and electroporation experiments.
Direct transfer of resistance into azide-resistant E. coli J53 and ciprofloxacin-resistant P. aeruginosa PU21 was attempted as previously reported (20). Plasmids were introduced by electroporation into E. coli DH10B (25) and P. aeruginosa KG2505 (23, 31) using a Gene Pulser II (Bio-Rad). The mating cultures were plated onto TS agar plates containing ticarcillin (100 μg/ml) and sodium azide (100 μg/ml) or ciprofloxacin (15 μg/ml), and the electroporants were plated onto on TS agar plates containing ticarcillin (100 μg/ml).
Nucleic acid extractions.
Recombinant plasmids were extracted by using Qiagen plasmid Mini-Midi kits (Qiagen, Courtaboeuf, France), whereas natural plasmids were extracted according to the Kieser technique (16). Plasmid extracts were subsequently analyzed by electrophoresis on a 0.7% agarose gel. Total DNA from P. aeruginosa isolates was extracted as described previously (25).
Cloning experiments and analysis of recombinant plasmids.
Unless specified, standard molecular techniques were used (30). Whole-cell DNAs were extracted as described previously (25). The ligation products of HindIII-digested total DNA of P. aeruginosa MUS into HindIII-restricted pBKCMV were electroporated into E. coli DH10B, and selection was performed on TS agar plates containing amoxicillin (100 μg/ml) and kanamycin (30 μg/ml).
PCR screening and genetic environment of blaOXA-18.
Taq DNA polymerase was from Roche Diagnostics (Meylan, France). Standard PCR amplification experiments (20, 25) were attempted. Primers specific for genes coding for the β-lactamases OXA-10, OXA-20, OXA-18, TEM, SHV, PER, VEB, GES, and BEL have been detailed previously (20, 21, 22, 25, 28, 27). The PCR products were purified by using QIAquick columns (Qiagen).
PCR experiments were performed on an ABI 2720 thermocycler (Applied Biosystems, Les Ulis, France) using laboratory-designed primers (Table 1) as previously described (25). PCR products were then analyzed on an agarose gel and sequenced.
Mapping the blaOXA-18 transcription start site.
Reverse transcription and RACE (rapid amplification of cDNA ends) were performed with a 5′RACE system (version 2.0; Invitrogen). Then, 5 μg of total RNAs extracted from P. aeruginosa MUS (Qiagen RNeasy Maxi kit) was used to determine the blaOXA-18 initiation site of transcription.
After a reverse transcription step with gene-specific primer OXA-GSP1 and reverse transcriptase, the cDNA was tailed with cytosines by using the terminal deoxynucleotidyltransferase and was subsequently amplified with another gene-specific primer, OXA-GSP2 combined with an oligo-dG adapter primer provided with the kit. This PCR product was used as a template for a nested PCR assay with a second adapter-primer (provided with the kit) and OXA-GSP3 primer located at the very beginning of blaOXA-18 or OXA-GSP4 primer located at the very beginning of aac6′-Ib gene (Table 1). The amplicon obtained was directly sequenced. The transcription initiation site was determined as the first nucleotide following the sequence of the adapter primer.
DNA sequencing and protein analysis.
Both strands of the PCR products and of the cloned DNA fragment of recombinant plasmid pJOA-1 were sequenced by using laboratory-designed primers with an automated sequencer (ABI Prism 3100; Applied Biosystems). The nucleotide and the deduced protein sequences were analyzed by using software available at the National Center of Biotechnology Information website (http://www.ncbi.nlm.nih.gov).
Genotyping and hybridization.
PFGE was performed using SpeI (Amersham Biosciences) as previously described (10). SpeI macrorestriction patterns were interpreted according to the recommendations of Tenover et al. (32).
DNA-DNA hybridizations were performed as described by Sambrook et al. (30), with a Southern transfer of a PFGE agarose gel that contained total DNA of P. aeruginosa isolates. The probe consisted of a 600-bp PCR-generated fragment from recombinant plasmid pJOA-1 and was internal to the blaOXA-18 gene. Labeling of the probe and signal detection were carried out by use an ECL nonradioactive labeling and detection kit (Amersham Biosciences) according to the manufacturer's instructions.
IEF analysis.
β-Lactamase extracts were prepared as described previously (10, 20) and subjected to analytical isoelectric focusing (IEF) on a pH 3.5 to 9.5 ampholine polyacrylamide gel (Amersham Biosciences), as described elsewhere (25).
Nucleotide sequence accession number.
The nucleotide sequences reported in the present study have been assigned to the EMBL/GenBank nucleotide database under the accession no. EU503121. The nucleotide sequences of the ISCR insertion sequences reported in the present study have been submitted to M. Toleman and assigned the number ISCR19.
RESULTS
Cloning of the blaOXA-18 gene from P. aeruginosa MUS.
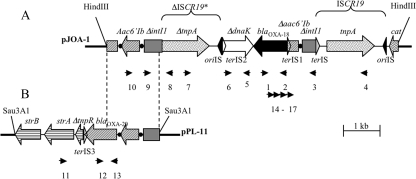
HindIII-restricted genomic-DNA of P. aeruginosa MUS was cloned into pBKCMV vector. Several E. coli transformants were obtained for the cloning experiment and selected on medium supplemented with kanamycin and amoxicillin. Two phenotypes were observed: an AmpC phenotype that was not further studied (data not shown) and an ESBL phenotype that corresponded to E. coli containing a recombinant plasmid, pJOA-1, with an 8.2-kb HindIII insert (Fig. 1) that was further analyzed.
FIG. 1.
Schematic representations of the genetic environment of the blaOXA-18 gene (A) and the blaOXA-20 gene (B) (22) in P. aeruginosa MUS. The coding regions are shown as boxes, with an arrow indicating the orientation of transcription. Black circles indicate the integron- and gene cassette-specific recombination sites attI and attC, respectively. Restriction sites that were used for cloning are indicated. Primers used for PCR-mapping experiments are indicated by small horizontal arrows (Table 1). Vertical dashed lines indicate the identity between the plasmids pJOA-1 and pPL11. Filled and empty triangles represent ISCR-specific sequences (oriISs are black and terISs have the color of the ORF they are located within).
Characterization of the genetic environment of the blaOXA-18 gene in P. aeruginosa MUS.
The nucleotide sequence of the ∼8.2-kb insert of plasmid pJOA-1 was determined and revealed several open reading frames (ORFs) (Fig. 1). The immediate genetic environment of blaOXA-18 gene was identical to that previously described (25), being upstream of a 3′-truncated aac6′-Ib (Δaac6′-Ib) gene cassette and downstream a 5′-truncated gene that codes for a putative chaperone protein DnaK that shares 79% sequence identity with a gene from Rhizobium etli CFN42 (80% amino acid identity) (9, 11, 25).
Further upstream of the Δaac6′-Ib gene cassette a class 1 integron-specific recombination site, attI, preceded by the 5′ end of an integrase gene, intI1, of class 1 integrons was identified (ΔintI1) (8, 9). The ΔintI1gene was interrupted by a novel insertion sequence of the ISCR family, termed ISCR19.
Downstream of ΔdnaK, a truncated copy of ISCR19 was found that lacked 300 bp, including its terIS and the 40 first amino acids of the transposase gene. This truncated copy shared 92% nucleotide identity with ISCR19 and was thus named ΔISCR19*. At the site of truncation, ΔISCR19* was fused to another truncated copy of the intI1 gene (ΔintΙ1) in opposite orientation (Fig. 1). Farther downstream, another copy of the aac6′-Ib gene cassette followed by the 5′ end of blaOXA-20 gene cassette was identified. The sequence downstream of blaOXA-20 was matched by PCR to be identical to that characterized on plasmid pPL11 (Fig. 1B) (22).
Sequence analysis of ISCR19 and ISCR19*.
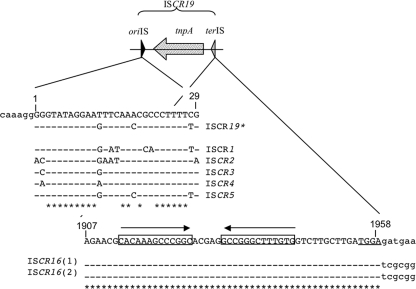
ISCR19 is 1,958 bp long and is delimited by two sequences: oriIS and terIS (Fig. 2). ISCR19 belongs to the ISCR3/ISCR5 group (88% nucleotide identity with ISCR5) and is structurally related to the ISCR16 elements found in the avian pathogenic E. coli plasmid pAPEC-01-R (14) (GenBank no. DQ517526) and in the Salmonella enterica plasmid sequence (36) (GenBank no. CP000604) (91% nucleotide identity). The oriIS sequence is located 245 bp downstream of the stop codon of the transposase gene. This sequence is conserved and matches those of well-characterized ISCRs (35) (Fig. 2).
FIG. 2.
Alignment of the oriIS and terIS of ISCR19 with that of ISCR elements. oriIS and terIS are the initiation and termination sites of ISCR19 transposition, respectively, and tnpA represents the transposase gene. Identical bases compared to ISCR19 are indicated by dashes, and conserved bases found in all of the sequences are indicated by asterisks. (Top panel) Alignment of the first 29 bp of the various ISCR elements with those of ISCR19, showing oriIS. (Bottom panel) Alignment of terIS of ISCR19 with the sequences found at the equivalent termini of ISCR16. IRs are underlined. Accession numbers are given in the text.
The terIS sequence is often difficult to determine precisely for ISCRs, since the flanking sequences are often identical or deleted (35). In the case of ISCR19, similar elements, such as ISCR16 (sharing 90% nucleotide identity), have been characterized on naturally occurring plasmids (14, 36). Alignment of the region encompassing the beginning of the transposase gene up to the flanking sequences revealed sequence identity until a TGGA motif (Fig. 2), thus suggesting the likely end of the ISCR19/ISCR16 elements. Furthermore, as suggested for terIS sequences of other ISCR elements (35), the ISCR19/ISCR16 terIS sequences contain a 13-bp IR region. To date, ISCR19 is associated only with the blaOXA-18 gene in a manner similar to that of ISCR5, which is exclusively associated with OXA-45, another OXA-ESBL (35).
ISCR19* is 1,663 bp long and is delimited by an oriIS that is very close to that of ISCR19 (3 changes out of 29). The oriIS sequence is located 259 bp downstream of the stop codon of the transposase gene. This sequence is conserved and matches well with those of well-characterized ISCRs (35) (Fig. 2).
Mapping of the blaOXA-18 transcription start site.
Computer-assisted promoter analysis had suggested that the promoter upstream of the blaOXA-18 gene was 84 bp from the translational start site (25). However, using 5′RACE PCR experiments, we could not confirm this site; instead, the site of initiation of transcription of the blaOXA-18 gene was mapped in P. aeruginosa MUS to be 690 bp upstream of the translational start codon, corresponding to the class 1 Pc (formerly known as Pant) promoter located in the integron (7, 8; data not shown).
Identification of the blaOXA-18 gene in Belgian P. aeruginosa isolates.
ESBL-producing ceftazidime-resistant isolates of P. aeruginosa were isolated from respiratory tract specimens of three patients over a 9-month period in 2006 and 2007 at the Saint-Luc University Hospital in Brussels, Belgium. In the three patients, the P. aeruginosa isolates were recovered at least 48 h after admission, suggesting that the organisms were hospital acquired. The patients had been hospitalized in different wards at different time periods, and no common source or diagnostic invasive procedures could be found. The three isolates were resistant to ticarcillin, aztreonam, and ceftazidime, but they were susceptible to ticarcillin-clavulanate, piperacillin, and piperacillin-tazobactam and intermediate to meropenem and cefepime according to Clinical and Laboratory Standards Institute breakpoints. A synergy image could be observed for the three isolates between ceftazidime, cefepime, and ticarcillin-clavulanic acid on MH agar only with disks placed 2 cm apart and on cloxacillin-containing plates. PCR amplification identified blaOXA-18 and blaOXA-20 in these isolates. Sequencing of the PCR fragments revealed 100% sequence identity with the previously described blaOXA-18 and blaOXA-20 genes (22, 25). These results were confirmed by IEF analysis. β-Lactamase extracts of cultures of P. aeruginosa 1-52, 1-63, 1-22, and MUS that were subjected to analytical IEF expressed three β-lactamases with pI values of 5.5, 6.0, and 8.6, respectively, a finding consistent with those of β-lactamases OXA-18, OXA-20, and AmpC, respectively, from P. aeruginosa (23).
Plasmid content and transfer of resistance.
No plasmid DNA was detected in P. aeruginosa 1-52, 1-63, and 1-22 isolates despite repeated analyses. Transfer by electroporation of the ticarcillin resistance marker from P. aeruginosa 1-52, 1-63, 1-22, and MUS isolates to E. coli J53 or P. aeruginosa PU21 failed, suggesting a chromosomal location for the β-lactamase genes. These results are in agreement with those found previously for P. aeruginosa MUS (25).
Strain typing and blaOXA-18-blaOXA-20 genetic environment.
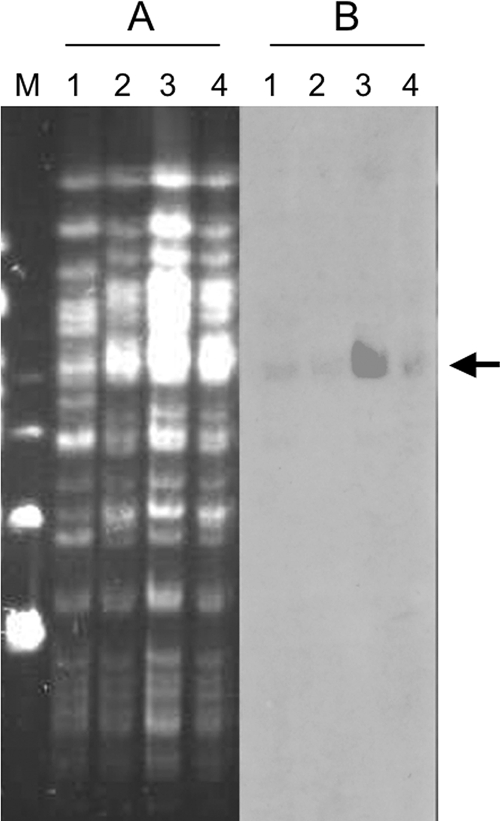
PFGE analysis using the SpeI restriction enzyme revealed only slight differences between the three Belgian P. aeruginosa isolates, thus suggesting their epidemiological relationship. These strains differed, however, more extensively from P. aeruginosa MUS (more than seven-band differences [Fig. 3A]). The SpeI-restricted DNA separated on the PFGE gel was transferred onto a nylon membrane and hybridized with an internal blaOXA-18-specific probe. A hybridization signal of high molecular weight (ca. 200 kb) (Fig. 3B) was detected for all P. aeruginosa isolates, indicating that the genomic DNA fragment carrying the blaOXA-18 gene is likely the same in all isolates.
FIG. 3.
Molecular comparison of blaOXA-18-producing P. aeruginosa isolates. (A and B) PFGE with SpeI-restricted DNA (A) and blaOXA-18 hybridization of the SpeI PFGE gel (B). Lane 1, P. aeruginosa MUS; lane 2, P. aeruginosa 1-63; lane 3, P. aeruginosa 1-22; lane 4, P. aeruginosa 1-52. Molecular weight markers (lane M) correspond to the lambda ladder (Bio-Rad). The arrow indicates the 200-kb band that hybridizes with the OXA-18 specific probe.
Using the genetic environment determined for the blaOXA-18 gene in P. aeruginosa MUS, primers were designed to amplify the genetic environment in the Belgian strains (Fig. 1). Similar-sized PCR products were obtained for all of the P. aeruginosa isolates (data not shown), and subsequent sequencing of these fragments revealed identical genetic environments.
DISCUSSION
The most common mechanisms of resistance to oxyimino-cephalosporins in P. aeruginosa correspond to overexpression of the AmpC chromosomal enzyme (18). The prevalence of ESBLs in P. aeruginosa is variable depending on the type of ESBL and the geographic origin (10, 17, 21). The prevalence of OXA-ESBLs is difficult to estimate, most of these enzymes being reported in single P. aeruginosa clinical isolates from Turkey, France, and Korea (21). OXA-18 had been identified previously from a single P. aeruginosa MUS clinical isolate in France in 1996 (25) and 10 years later in Tunisia in 2006, during an outbreak involving a P. aeruginosa clone that was unrelated to P. aeruginosa MUS (15) In the present study, OXA-18-producing isolates from Belgium were also detected and compared to the prototype P. aeruginosa MUS strain.
The three OXA-18-producing P. aeruginosa isolates were gathered over a 9-month period from patients located in three different wards of the same hospital. The patients had not been in contact with one another during hospitalization and had no apparent source exposure or invasive procedure in common. The three P. aeruginosa strains were deemed to be colonizing rather than pathogenic organisms (data not shown). The prevalence of this OXA-18-producing P. aeruginosa strain in the hospital is not known, but it might be present on an endemic basis, probably due to underdetection, especially when chromosomal cephalosporinase is overexpressed.
The failure to identify a plasmid suggested that the gene encoding OXA-18 is chromosomally mediated, which is in agreement with previous studies (15, 22, 25). The Belgian P. aeruginosa isolates were closely related to each other as determined by PFGE analysis, suggesting a strong epidemiological link between these isolates. The three Belgian strains were different from P. aeruginosa MUS. However, detailed analysis of the PFGE patterns revealed some genetic relatedness between P. aeruginosa MUS and the Belgian isolates, especially the blaOXA-18-containing fragment that is conserved in all of the strains. Moreover, sequencing analysis confirmed that the immediate genetic environments of blaOXA-18 and blaOXA-20 were identical in French and Belgian strains. The recently identified OXA-18-producing P. aeruginosa isolates from Tunisia were blaOXA-20 negative (15) (but positive for either SHV-1 or, less frequently, TEM-1) and genetically different from P. aeruginosa MUS, thus suggesting that at least two OXA-18-producing P. aeruginosa clones are currently identified worldwide.
The genetic environment of blaOXA-18 was different from that of most oxacillinase genes, since this gene was not located in an integron, in contrast to the blaOXA-10, blaOXA-2, and blaOXA-1 genes of P. aeruginosa (19, 21), and it was not composite transposon-borne, such as the blaOXA-48 gene of K. pneumoniae (3) and the blaOXA-23 and blaOXA-58 genes of A. baumannii (5, 26). Rather, blaOXA-18 was associated with ISCR elements. ISCR elements have been found associated with many resistance genes, including β-lactam resistance genes of different Ambler classes (2). The only other example of an oxacillinase gene associated with ISCR elements is blaOXA-45 gene, which is associated with the ISCR5 element. Interestingly, OXA-45 is another OXA-ESBL and ISCR19, like ISCR5, belongs to a subset of ISCR3-type elements (35). blaOXA-18 was surrounded by two copies of ISCR19-like elements that share 92% sequence identity. It is likely that these elements derive from a common ancestor and have diverged (35). Insertions of ISCR have been reported to provide downstream inserted genes with a promoter sequence (35). Here, the element seems not to fulfill this function, but ISCR19, by mobilizing the blaOXA-18 gene into the aac6′-Ib gene, allowed its expression by the 5′ conserved sequence (CS)-located promoter Pc (formerly known as Pant) that is equally well recognized in P. aeruginosa, A. baumannii, and Enterobacteriaceae (3, 7, 8).
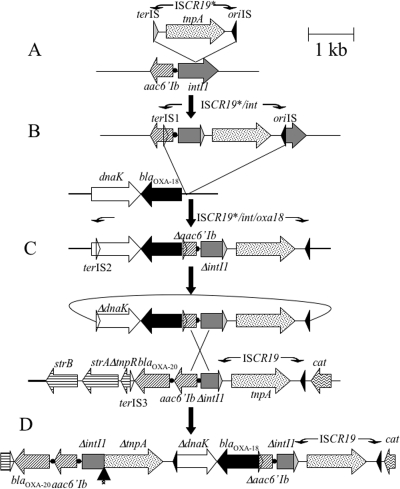
ISCR19 may have likely been at the origin of blaOXA-18 gene mobilization. At some time in the past, the ISCR19* transposed into the 5′CS (integrase gene, intI1) of a class 1 integron (Fig. 4A). From this point on, ISCR19* is able to mobilize part of the integrase gene and any antibiotic resistance gene cassettes therein by an IS91-like RC mechanism possibly recognizing various putative termination sequence (terIS-1, terIS-2 or terIS-3 in Fig. 1 and 4), similar or not to its original and cognate terIS. It is also possible that ISCR19* does not possess an intrinsic termination site and, accordingly, terminates transposition randomly, thereby mobilizing varied lengths of 5′-located (upstream) DNA. As suggested for IS91-like elements (6) (Fig. 4C), free circular intermediates could be generated, carrying ISCR19 and sequences adjacent to it and distal to oriIS, including at least the truncated 5′CS and the linked resistance gene. These circular entities can then in turn insert by transposition or be rescued by homologous recombination into either the 5′CS of conventional class 1 integrons or that of an integron-ISCR19-like variant, which seemed to be the case here. The arrangement that would arise from the recombination event (Fig. 4C and D) is that there would be a direct duplication of the ISCR element following the duplication of the Δ5′CS-Δaac6′-Ib.
FIG. 4.
Proposed model of ISCR19-mediated mobilization of blaOXA-18 and genesis of a blaOXA-18-containing intI1 complex class 1 integron. The construction of blaOXA-18-containing complex class 1 integrons has been inspired from the model proposed by Toleman et al. Several steps are necessary. (A) Insertion of ISCR19* into a class 1 integrase gene. Aberrant RC replication of the ISCR19* element (inserted into the int1 gene) generates a transposition intermediate starting at oriIS, ending at another terIS, and then its cognate terIS at terIS1, located inside the aac6′-Ib gene. (B) This intermediate then transposes adjacent to the blaOXA-18 gene in another location. (C) A second aberrant RC replication event produces circular intermediates that now include the blaOXA-18 gene. (D) These circular intermediates may then be rescued by recombination events between aac6′-Ib or integrase genes on another a class 1 integron already including a copy of ISCR19, generating the blaOXA-18-containing complex integron. Boxes represent the ORFs of the genes, with arrows indicating the direction of their transcription. The transposase gene of the ISCR19 elements are dotted, the integrase gene is shaded in gray, and the blaOXA-18 gene is black. The integrase-specific recombination sites are indicated as black dots. oriIS is represented as an black triangle, terIS is represented as a gray triangle, and terIS1, -2, and -3 represent secondary terIS (open triangles). The vertical arrow in panel D indicates the deletion event that occurred fusing the 5′CS sequence with that of tnpA of ISCR19*.
While ISCR1 or ISCR3 and ISCR4 elements mobilize adjacent DNA sequences that are subsequently rescued by homologous recombination via flanking 3′CS sequences or via flanking groEL sequences, respectively (35), ISCR19 mobilizes adjacent DNA sequences by homologous recombination via flanking Δ5′CS-Δaac6′-Ib sequences. ISCR19* appeared to have lost its original terIS sequence (Fig. 4D), possibly by a deletion event linking intI1 to the 5′ end of the transposase gene. A similar deletion appeared with ISCR4, which has lost its original terIS sequence, possibly by a deletion event linking groEL to the 5′ end of ISCR4 (35). In our case, however, due to interruption of the transposase gene, it is likely that ISCR19* can only function when a transposase is provided in trans. It is, however, not possible to clearly position this deletion event in our transposition model; the deletion could have occurred early and then the transposase would have been trans-complemented by the intact ISCR19-copy, or it could have occurred at a very late stage.
The present study highlights the spread of OXA-18-producing P. aeruginosa isolates in a Belgium hospital and suggests that their prevalence might be underestimated due to their underdetection. Furthermore, the present study identified a novel ISCR element, ISCR19 associated with the blaOXA-18 resistance gene and likely at the origin of its genetic mobilization. Clinically, the most interesting aspect of ISCR elements is that they are increasingly being reported with powerful resistance determinants, such as metallo-β-lactamases and now OXA-ESBLs.
Acknowledgments
We thank C. Bauraing for assistance in preliminary PCR experiments. We are grateful to T. Walsh and M. Toleman for helpful discussions.
This study was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539); the Université Paris XI, Paris, France; and mostly by the European Community (6th PCRD, LSHM-CT-2005-018-705).
Footnotes
Published ahead of print on 28 July 2008.
REFERENCES
- 1.Al Naiemi, N., B. Duim, and A. Bart. 2006. A CTX-M extended-spectrum beta-lactamase in Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Med. Microbiol. 55:1607-1608. [DOI] [PubMed] [Google Scholar]
- 2.Ambler, R. P., A. F. Coulson, J.-M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, D., T. Naas, C. Héritier, L. Poirel, and P. Nordmann. 2006. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of beta-lactam resistance genes. J. Bacteriol. 188:6506-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Corvec, S., L. Poirel, T. Naas, H. Drugeon, and P. Nordmann. 2007. Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-23 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Pilar Garcillán-Barcia, M., I. Bernales, M. V. Mendiola, and F. de la Cruz. 2001. Single-stranded DNA intermediates in IS91 rolling-circle transposition. Mol. Microbiol. 39:494-501. [DOI] [PubMed] [Google Scholar]
- 7.Depardieu, F., I. Podglajen, R. Leclercq, E. Collatz, and P. Courvalin. 2007. Modes and modulations of antibiotic resistance gene expression. Clin. Microbiol. Rev. 20:79-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluit, A. C., and F. J. Schmitz. 2004. Resistance integrons and super-integrons. Clin. Microbiol. Infect. 10:272-288. [DOI] [PubMed] [Google Scholar]
- 9.Galimand, M., T. Lambert, G. Gerbaud, and P. Courvalin. 1993. Characterization of the aac(6′)-Ib gene encoding an aminoglycoside 6′-N-acetyltransferase in Pseudomonas aeruginosa BM2656. Antimicrob. Agents Chemother. 37:1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girlich, D., T. Naas, A. Leelaporn, L. Poirel, M. Fennewald, and P. Nordmann. 2002. Nosocomial spread of the integron-located veb-1-like cassette encoding an extended-spectrum β-lactamase in Pseudomonas aeruginosa in Thailand. Clin. Infect. Dis. 34:603-611. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez, V., R. I. Santamaria, P. Bustos, I. Hernandez-Gonzalez, A. Medrano-Soto, G. Moreno-Hagelsieb, S. C. Janga, M. A. Ramirez, V. Jimenez-Jacinto, J. Collado-Vides, and G. Davila. 2006. The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc. Natl. Acad. Sci. USA 103:3834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall, L. M., D. Livermore, D. Gur, M. Akova, and H. E. Akalin. 1993. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:1644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, T. J., Y. M. Wannemeuhler, J. A. Scaccianoce, S. J. Johnson, and L. K. Nolan. 2006. Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli isolates. Antimicrob. Agents Chemother. 50:3929-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalai Blagui, S., W. Achour, M. S. Abbassi, M. Bejaoui, A. Abdeladhim, and A. Ben Hassen. 2007. Nosocomial outbreak of OXA-18-producing Pseudomonas aeruginosa in Tunisia. Clin. Microbiol. Infect. 13:794-800. [DOI] [PubMed] [Google Scholar]
- 16.Kieser, T. 1984. Factors affecting the isolation of cccDNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 17.Kolayli, F., G. Gacar, A. Karadenizli, A. Sanic, H. Vahaboglu, and the Study Group. 2005. PER-1 is still widespread in Turkish hospitals among Pseudomonas aeruginosa and Acinetobacter spp. FEMS Microbiol. Lett. 249:241-245. [DOI] [PubMed] [Google Scholar]
- 18.Livermore, D. M., and N. Woodford. 2006. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas, and Acinetobacter. Trends Microbiol. 14:413-420. [DOI] [PubMed] [Google Scholar]
- 19.Naas, T., and P. Nordmann. 1999. OXA-type beta-lactamases. Curr. Pharmaceut. Des. 5:865-879. [PubMed] [Google Scholar]
- 20.Naas, T., D. Aubert, T. Lambert, and P. Nordmann. 2006. Complex genetic structures with repeated elements, a sul-type class 1 integron, and the blaVEB extended-spectrum β-lactamase gene. Antimicrob. Agents Chemother. 50:1745-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naas, T., L. Poirel, and P. Nordmann. 2008. Minor extended-spectrum β-lactamases. Clin. Microbiol. Infect. 14(Suppl. 1):42-52. [DOI] [PubMed] [Google Scholar]
- 22.Naas, T., W Sougakoff, A Casetta, and P. Nordmann. 1998. Molecular characterization of OXA-20, a novel class D β-lactamase, and its integron from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:2074-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto, K., N. Gotoh, and T. Nishino. 2001. Pseudomonas aeruginosa reveals high intrinsic resistance to penem antibiotics: penem resistance mechanisms and their interplay. Antimicrob. Agents Chemother. 45:1964-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippon, L. N., T. Naas, A.-T. Bouthors, V. Barakett, and P. Nordmann. 1997. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2188-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel, L., and P. Nordmann. 2006. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poirel, L., L. Brinas, A. Verlinde, L. Ide, and P. Nordmann. 2005. BEL-1, a novel clavulanic acid-inhibited extended-spectrum β-lactamase, and the class 1 integron In120 in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3743-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirel, L., P. Gerome, C. De Champs, J. Stephanazzi, T. Naas, and P. Nordmann. 2002. Integron-located OXA-32 gene cassette encoding an extended-spectrum variant of OXA-2 β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:566-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recchia, G. D., H. W. Stokes, and R. M. Hall. 1994. Characterization of specific and secondary recombination sites recognized by the integron DNA integrase. Nucleic Acids Res. 22:2071-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Smith, A. W., and B. H. Iglewski. 1989. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 17:10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenover, F. C., R. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavakoli, N., A. Comanducci, H. M. Dodd, M. C. Lett., B. Albiger, and P. Bennett. 2000. IS1294, a DNA element that transposes by RC transposition. Plasmid 44:66-84. [DOI] [PubMed] [Google Scholar]
- 34.Toleman, M. A., K. Rolston, R. N. Jones, and T. R. Walsh. 2003. Molecular and biochemical characterization of OXA-45, an extended-spectrum class 2d′ β-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:2859-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toleman, M. A., P. M. Bennett, and T. R. Walsh. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welch, T. J., W. F. Fricke, P. F. McDermott, D. G. White, M. L. Rosso, D. A. Rasko, M. K. Mammel, M. Eppinger, M. J. Rosovitz, D. Wagner, L. Rahalison, J. E. Leclerc, J. M. Hinshaw, L. E. Lindler, T. A. Cebula, E. Carniel, and J. Ravel. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS ONE 2:E309. [DOI] [PMC free article] [PubMed] [Google Scholar]