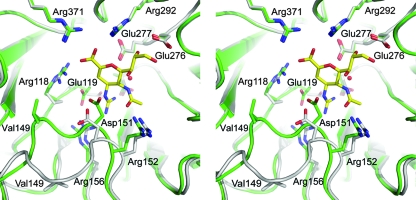
FIG. 5.
Stereo view of the active sites of the superimposed zanamivir-bound (green) and unbound (gray) structures of 18NA. Conserved charged residues, Arg118, Asp151, Arg152, Arg224, Glu276, Arg292, and Arg371, as well as the structurally conserved water molecule involved in the direct interactions with the inhibitor, are shown in ball-and-stick representation. Val 149 in the 150 loop, in which the major conformational changes occur upon inhibitor binding, is also highlighted in stick presentation to illustrate the extent of the loop rearrangement.