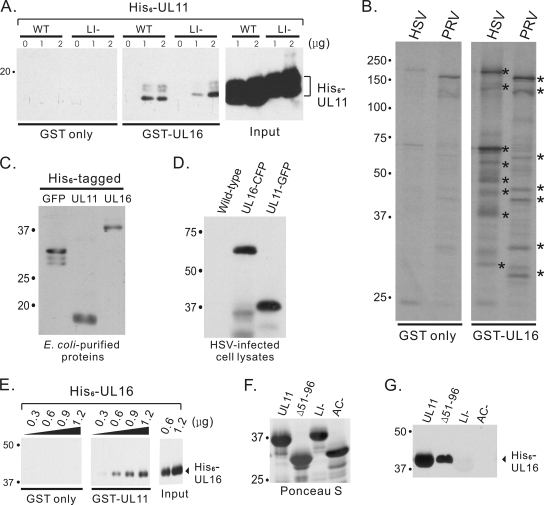
FIG. 2.
Direct interaction assays with UL11 and UL16. (A) The indicated amounts of His6-UL11 proteins were incubated with purified GST only or GST-UL16. Bound proteins were separated by SDS-PAGE and detected by immunoblotting using a monoclonal antibody specific for the His6 tag. WT, wild type. (B) To examine the abilities of purified GST or GST-UL16 to pull down virus-specific proteins, they were incubated with radiolabeled HSV- or PRV-infected cell lysates. Proteins bound were separated by SDS-PAGE, and radiolabeled proteins were detected by autoradiography. Examples of virus-specific proteins pulled down by GST-UL16 are indicated with asterisks. (C and D) Immunoblot analyses were used to show the specificity of rabbit anti-His6-GFP serum for various bacteria-expressed His6-tagged proteins (C) and viral proteins made by wild-type and recombinant HSV strains (D). (E) The indicated amounts of His6-UL16 proteins were incubated with purified GST only or GST-UL11. Bound proteins were separated by SDS-PAGE and detected by immunoblotting. (F and G) Aliquots (1.8 μg) of His6-UL16 protein were incubated with approximately equal amounts of GST-UL11 or mutants (Δ51-96 or LI or AC mutants) as estimated from a Ponceau S-stained gel (F), and bound proteins were analyzed by immunoblotting (G).