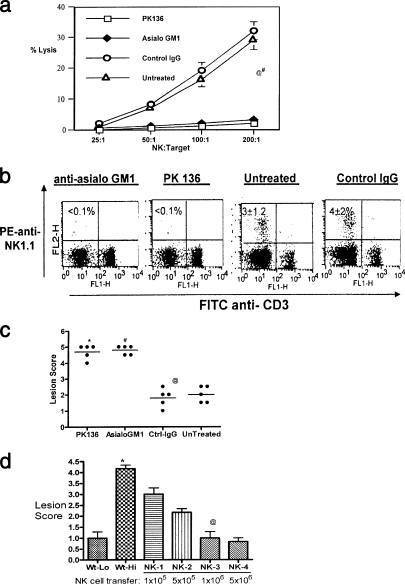
FIG. 1.
Lack of NK cells contributes to susceptibility, while supplementation enhances resistance. (a) Mice given anti-asialo-GM1 and anti-NK 1.1 antibodies lack detectable NK cell lytic activity. Sample animals from groups administered anti-asialo-GM1 or control IgG were sacrificed 48 h later. Spleen cells were analyzed for in vitro NK cell cytotoxic activity. A suspension of 107/ml of spleen cells was serially diluted with 100 ml in each triplicate well of a 96-well round-bottomed plate. Target 51Cr-labeled YAC-1 mouse lymphoma cells were added to give a effector/target cell ratio of 200, 100, 50, or 25. After 4 h of incubation at 37°C, supernatants containing released 51Cr were collected and counted with an automatic scintillation counter. Specific lysis was calculated as (experimental release − spontaneous release)/(total release − spontaneous release) × 100%, where spontaneous release was derived from wells without effectors and total release from wells with 3% Triton-X added to it. @, asialo-GM1 versus control IgG or untreated, P < 0.0001; #, PK136 versus control IgG and untreated controls, P < 0.0001. (b) Depletion of NK cells by anti-NK1.1 and asialo-GM1 antibodies. B6 mice given anti-asialo-GM1 and PK136 were analyzed for the absence of NK cells by flow cytometry. Single-cell suspensions of spleens were stained with anti-NK1.1 (PE) and anti-CD3 (FITC). The plots represent data obtained from one mouse in each group. The numbers within the plot are the means ± SDs for five mice in each group. The controls included untreated mice and mice given control IgG. Cells that are CD3− NK1.1+ are taken to be the NK cells. (c) Early onset of lesions upon zosteriform challenge in NK-depleted mice. Zoster challenge experiments were performed as described elsewhere (20). Before challenge, the left flank area was depilated by a combination of hair clipping and use of the chemical Nair (Carter-Wallace, New York, NY). The animals were anesthetized with avertin, and scarifications were made in a ∼4-mm2 area. To such scarifications, 10 μl containing 104 PFU of HSV-1 (strain 17) was added and gently massaged. Animals were inspected daily for the development of zosteriform ipsilateral lesions, general behavior changes, encephalitis, and mortality. The severity of the lesions was scored as follows: 1+, vesicle formation; 2+, local erosion and ulceration of the local lesion; 3+, mild to moderate ulceration; 4+, severe ulceration, hind limb paralysis, and encephalitis; and 5+, ultimate death (*, mice that were moribund and hence euthanized). The experiments were repeated three times with five mice in each group, and the outcomes were similar. The lesion scores of all mice within a group at day 10 postchallenge from one such experiment are shown. @, Asialo-GM1 versus control IgG and untreated, P < 0.0001; #, PK136 versus control IgG and untreated, P < 0.0001. (d) NK cell transfer augments protection against HSV. Wild-type B6 mice were divided into six groups of three mice each. Groups 1 and 2 were challenged (zosteriform) with low (5 × 103) (Wt-Lo) and high (1 × 105) (Wt-Hi) doses of virus, respectively. Groups 3 to 6 (NK-1, -2, -3, and -4, respectively) were adoptively transferred with increasing numbers of purified NK cells as indicated 15 h before challenge with the high dose of virus. The animals were handled as described for panel c and lesion scores recorded. The experiment was repeated three times with three mice per group. Data collected on day 10 postchallenge in one such experiment are shown. *, animals in this group were moribund and hence sacrificed. @, statistically significant difference between NK-3 (1 million NK cells) and Wt-Hi (P < 0.05).