FIG. 7.
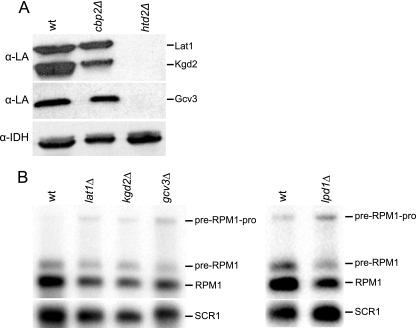
The FAS II pathway is the sole source of octanoic acid for protein lipoylation. (A) Western blot analysis of lipoylated proteins in a FAS II mutant strain. Mitochondrial extracts from the wild-type strain (wt), the cbp2Δ strain, a respiration-deficient control strain, and the htd2Δ strain were analyzed by Western blotting using an antibody directed against lipoic acid (α-LA). Lat1 and Kgd2 are the lipoylated E2 proteins of PDH and α-KDH, respectively, and Gcv3 is the lipoylated H protein of GC. Antiserum IDH (α-IDH) was used as a loading control. (B) Northern blot analysis of pre-RPM1-pro processing in the lat1Δ, kgd2Δ, and gcv3Δ strains, as well as in the lpd1Δ strain, was done using the RPM1 probe. Lpd1 is a common subunit shared by the three lipoic acid-dependent enzyme complexes. SCR1 was used as the loading control.