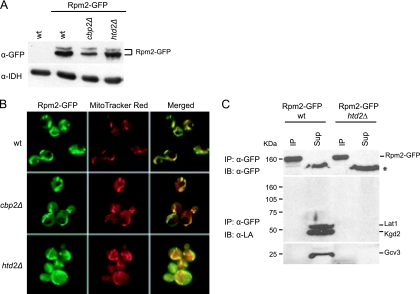
FIG. 8.
Analysis of Rpm2-GFP. Strains used were Rpm2-GFP wild-type, Rpm2-GFP cbp2Δ, a respiration-deficient control strain, and Rpm2-GFP htd2Δ. (A) Rpm2-GFP protein levels were analyzed by a Western blot of mitochondrial extracts, using an antibody against GFP (α-GFP) to detect Rpm2-GFP. IDH (antiserum α-IDH) was used as a loading control. (B) Rpm2-GFP is localized to mitochondria. Cells harvested at mid-log phase were analyzed by fluorescence microscopy. MitoTracker Red was added to the cultures 45 min before harvesting. (C) Rpm2-GFP is not lipoylated. Proteins from mitochondrial matrix fractions from Rpm2-GFP wild-type (wt) and Rpm2-GFP htd2Δ were immunoprecipitated (IP) with anti-GFP antibody and protein A-coupled beads. Nonimmunoprecipitated protein (Sup) was trichloroacetic acid precipitated, and 30% of both the IP and Sup fractions were loaded onto a 7 to 17% gradient SDS-polyacrylamide gel. Proteins were immunoblotted (IB) with anti-lipoic acid antibody (α-LA, lower panels). The blot was stripped and incubated with anti-GFP antibody (α-GFP, upper panel). The asterisk marks a nonspecific background band.