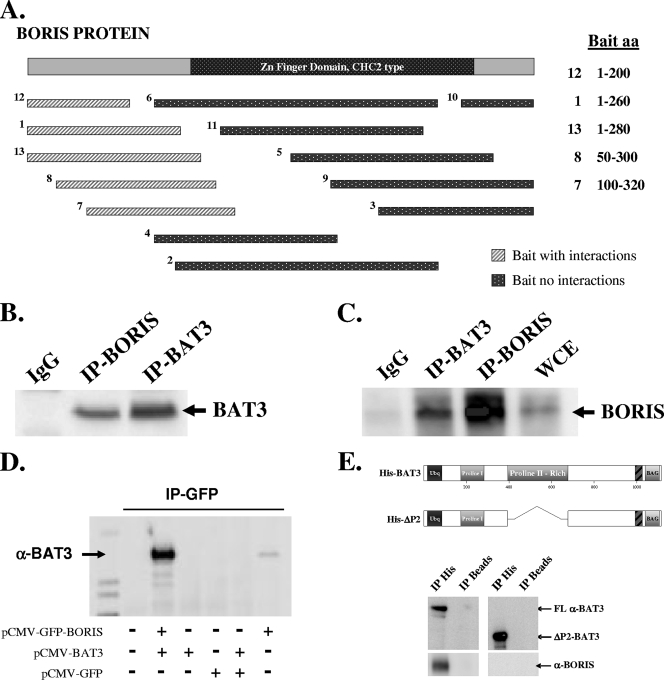
FIG. 1.
BAT3 physically interacts with BORIS in HCT116 cells. (A) Yeast two-hybrid design. Diagram of 13 bait sequences of BORIS used to identify binding partners. Light hatched rectangles represent baits that captured binding partners (Table 1), while dark dotted rectangles represent baits with no binding partners (see methods in the supplemental material). (B) BAT3 binds to BORIS in vitro. HCT116 cell lysates were subjected to IP with an anti-BORIS antibody (see Fig. S1 and S2 in the supplemental material), followed by Western analysis with an anti-BAT3 antibody. IgG, immunoglobulin G. (C) HCT116 cell lysates were subjected to IP with an anti-BAT3 antibody, followed by Western analysis with an anti-BORIS antibody. (D) COS-7 cells were transfected with pCMV-GFP, pCMV-GFP-BORIS, and/or pCMV-BAT3 and subjected to IP with an anti-GFP antibody. Immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis and blotted with an anti-BAT3 antibody. (E) BORIS binds to the BAT3 proline-rich region. HCT116 cell lysates were incubated with purified wild-type His-tagged BAT3 or His-tagged ΔP2-BAT3, which contains a deletion in the proline-rich region (aa 387 to 675 deleted). Lysates were subjected to IP with an anti-His antibody (Santa Cruz, Inc.), and the His tag IP products were resolved and immunoblotted with either an anti-BAT3 (top panel) or anti-BORIS (lower panel) antibody.