Abstract
How viral and host factors contribute to the severe pathogenicity of the H5N1 subtype of avian influenza virus infection in humans is poorly understood. We identified three clusters of differentially expressed innate immune response genes in lungs from H5N1 (A/Vietnam/1203/04) influenza virus-infected ferrets by oligonucleotide microarray analysis. Interferon response genes were more strongly expressed in H5N1-infected ferret lungs than in lungs from ferrets infected with the less pathogenic H3N2 subtype. In particular, robust CXCL10 gene expression in H5N1-infected ferrets led us to test the pathogenic role of signaling via CXCL10's cognate receptor, CXCR3, during H5N1 influenza virus infection. Treatment of H5N1-infected ferrets with the drug AMG487, a CXCR3 antagonist, resulted in a reduction of symptom severity and delayed mortality compared to vehicle treatment. We contend that unregulated host interferon responses are at least partially responsible for the severity of H5N1 infection and provide evidence that attenuating the CXCR3 signaling pathway improves the clinical course of H5N1 infection in ferrets.
According to the World Health Organization, the H5N1 subtype of avian influenza virus has caused over 380 cases of human illness and over 240 deaths from 2003 to date. Person-to-person transmission of H5N1 virus appears to be a rare event (20, 37). Current therapeutic strategies for human H5N1 infection are restricted to vaccination, preventative or early treatment with antiviral agents, and use of corticosteroids. While antiviral treatments may benefit patients if administered early in the disease and the currently available H5N1 vaccines appear to be effective in animal models and safe in humans, neither approach guarantees protection in the face of an influenza pandemic. Newly emergent strains may not match stockpiled vaccine strains, and the virus is likely to develop resistance to antiviral agents.
Recent studies have suggested that aberrant host immune responses to H5N1 during acute infection may be partially responsible for the severity of disease in humans (11, 35). Very few mechanistic data, however, are available regarding host responses to H5N1 infection in humans or animal models. Treatments designed to correct a dysregulated host response and associated immunopathology during H5N1 infection therefore warrant further consideration in animal models.
In this study, a functional genomics approach was utilized to identify potential patterns of immune dysregulation during acute H5N1 influenza virus infection in ferrets by comparison to H3N2 infection. Ferrets were infected intranasally with one of two different influenza A viruses originally isolated from humans: A/Vietnam/1203/04 (H5N1) and A/Panama/2007/99 (H3N2). Three clusters of differentially expressed innate immune response genes that were highly enriched in interferon (IFN) response genes (IRGs) were found by microarray analysis of gene expression in lungs from H5N1- versus H3N2-infected animals. In particular, CXCL10 gene expression was significantly upregulated in H5N1-infected ferret lungs throughout the course of the study relative to mock-infected and H3N2-infected ferret lungs. This result prompted us to evaluate modulation of CXCL10 activity via blockade of CXCR3 signaling for efficacy in improving the morbidity associated with H5N1 infection in ferrets and as a potential avenue for future therapeutic intervention in humans.
MATERIALS AND METHODS
Ferret experiments.
For the microarray study, 4- to 6-month old, neutered, descented, and influenza virus antibody-screened male fitch ferrets (Triple F Farms, Sayre, PA) were weighed and randomly assigned to one of three infection groups at the Southern Research Institute for intranasal instillation of either A/Vietnam/1203/04 H5N1 subtype (clade 1) or A/Panama/2007/1999 influenza virus at 1 × 106 50% egg infectious doses (EID50) or for phosphate-buffered saline (PBS) mock infection, in a total volume of 1 ml. Infected ferrets (n = 3/group) were euthanized at 2 and 4 days postinfection (dpi) and at the end point (6 dpi or upon scoring as moribund due to 25% weight loss). Note that one H5N1-infected ferret was euthanized at 5 dpi under the definition of the end point (see Fig. S1 in the supplemental material). Mock-infected animals were euthanized on the day of infection. Clinical signs were monitored daily from 2 dpi. Viral loads in nasal turbinates and lung tissue from influenza virus-infected ferrets at necropsy were assayed by methods described previously (44) with a limit of detection of 1.5 EID50/ml (log10). For the AMG487 treatment study, the drug was kindly provided by Amgen (San Francisco, CA) and administered at a suggested one-third of the daily dose used safely in mouse studies (38). Ferrets were infected with H5N1 as described above and received either 1.65 mg/kg AMG487 in a 3-ml volume of PBS (n = 8) or PBS vehicle (n = 9) intraperitoneally every 12 h starting at 24 h postinfection and continuing until the end point (euthanasia upon scoring as moribund due to 25% weight loss). Nasal washes at 1, 3, and 5 dpi and lungs at necropsy were assayed for viral load in the AMG487 treatment study by methods described previously (44) with a limit of detection of 1.5 EID50/ml (log10). Weight loss, activity, temperature, and arterial O2 saturation (SpO2) (pulse oximetry) were monitored daily from 2 dpi. Temperatures were monitored with subcutaneous transponders (IPTT-300; Biomedic Data Systems Inc, Seaford, DE).
Microarray analysis.
Lung sections (∼1 g) were obtained during necropsy and immediately homogenized in TriPure reagent, and total RNA was isolated according to the manufacturer's recommended protocol (Roche, Indianapolis, IN). RNA quality was assessed using a UV spectrophotometer and by formaldehyde gel electrophoresis. The quality of RNA isolated from a single H3N2-infected ferret's lung specimen collected at 4 dpi was insufficient for cDNA synthesis. Total RNA was amplified using MessageAMP kits (Ambion, Austin, TX). cRNA (15 μg) was labeled and hybridized to Affymetrix Canine 2.0 oligonucleotide arrays (Affymetrix, Santa Clara, CA). Canine arrays were chosen following analysis of 30 publically available ferret cDNA sequences and demonstration of high levels of homology (average of 89% identity) between canine and ferret nucleotide sequences (see Table S1 in the supplemental material). Probe-level analysis was performed using the Probe Logarithmic Intensity Error Estimate (PLIER) method in Array Assist V5.2 (Stratagene, La Jolla, CA). The raw intensity values for each target on the Affymetrix arrays were preprocessed with variance stabilization, log2 transformation, and normalization against the data sets for mock infection excluding elements with median signal intensities of <6 log2 units across samples. Sequential Student's t tests (time point versus mock) were used to identify genes significantly differentially expressed (P ≤ 0.05 and ≥2-fold change) for at least one time point and group during H3N2 and H5N1 infection in ferrets. These genes were combined with significantly differentially expressed genes between H3N2- and H5N1-infected ferrets (≥2-fold change for at least one time point and group and P ≤ 0.05) identified using the Extraction and Analysis of Differential Gene Expression (EDGE) software tool (24). Hierarchical clustering was performed using GeneLinker Platinum 4.6 (Improved Outcomes, Kingston, Canada) and the Euclidean geometrical average of samples algorithm. Ingenuity Pathway Analysis 5.0 (IPA) (Ingenuity Systems, Redwood City, CA) was used to select, annotate, and visualize genes by function and pathway (gene ontology). Additional gene annotation was provided by the Interferon Stimulated Gene Database (12) and the Universal Protein Resource (UNIPROT) (URL:www.expasy.org/uniprot).
qRT-PCR.
Quantitative real-time PCR (qRT-PCR) was performed on amplified RNA in triplicate using an ABI-Prism 7900HT and Sybr green master mix (Applied Biosystems, Foster City, CA). Primer sequences were as follows: for CXCL9, 5′-GGTGGTGTTCCTCTTTTGTTGAGT-3′ (forward) and 5′GGAACAGCGTCTATTCCTCATTG-3′ (reverse); for CXCL10, 5′-CTTTGAACCAAAGTGCTGTTCTTATC-3′ (forward) and 5′-AGCGTGTAGTTCTAGAGAGAGGTACTC-3′ (reverse); for FCN1, 5′-CACCAAGGACCAGGACAATGA-3′ (forward) and 5′-CACCAGGCCCCCTGGTA-3′ (reverse); for SERPING1, 5′-GCCTCTCAGAGCCTGTATGG-3′ (forward) and 5′-CTTCCACTTGGCACTCAGGT-3′ (reverse); and for STAT1, 5′-AGCCTTGCATGCCAACTCA-3′ (forward) and 5′-GCAGTCTCAACTTCACGGTGAA-3′ (reverse). Gene expression levels for each sample were normalized to ferret beta-actin levels in the same sample. The quality of cDNA obtained from a single H5N1-infected ferret's lung specimen collected at the end point was insufficient for qRT-PCR validation.
Histochemistry.
Lungs were routinely perfused, formalin fixed, and hematoxylin-and-eosin stained. Blinded serial sections were evaluated by a veterinary pathologist (P.V.T.).
Statistical methods.
Survival curves were evaluated by Kaplan-Meier and log rank analysis. Other clinical indicators were compared by two-way analysis of variance (ANOVA) (Prism V3; GraphPad Software, San Diego, CA). Other tests used were Student's t test or the Mann-Whitney (nonparametric) rank sum test for two independent populations using SPSS for Windows V13.0 software (SPSS Inc., Chicago, IL) where noted. A P value of ≤0.05 was considered significant with all methods.
Microarray data accession number.
Data sets are available publically at the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/) under accession number GSE9606.
RESULTS AND DISCUSSION
To identify host responses specific to H5N1 virus infection and further examine the molecular basis of the high degree of lung pathology associated with H5N1 infection, we infected ferrets with either the H5N1 or H3N2 subtype of influenza A virus (see Fig. S1 and S2 in the supplemental material) and obtained tissue samples for gene expression analysis at various time points postinfection. Ferrets were inoculated intranasally with 106 EID50 of either A/Vietnam/1203/04 (H5N1) or A/Panama/2007/99 (H3N2) and examined daily for clinical signs of disease, including loss of activity, nasal discharge and respiratory distress, neurological signs, weight loss, and temperature. Overall, the clinical symptoms we observed in H5N1-infected ferrets were very consistent with previous studies using this particular virus strain (A/Vietnam/1203/04) or its reverse-genetics-derived recombinant form (15, 31). At 2 and 4 dpi and at the end point, ferrets were euthanized and lung tissue removed for RNA purification for gene expression analysis. RNA was quantified and assessed for integrity, and equal quantities of lung total RNA from each ferret were amplified and hybridized to oligonucleotide arrays. The resulting gene expression data were normalized directly to those for mock-infected ferrets.
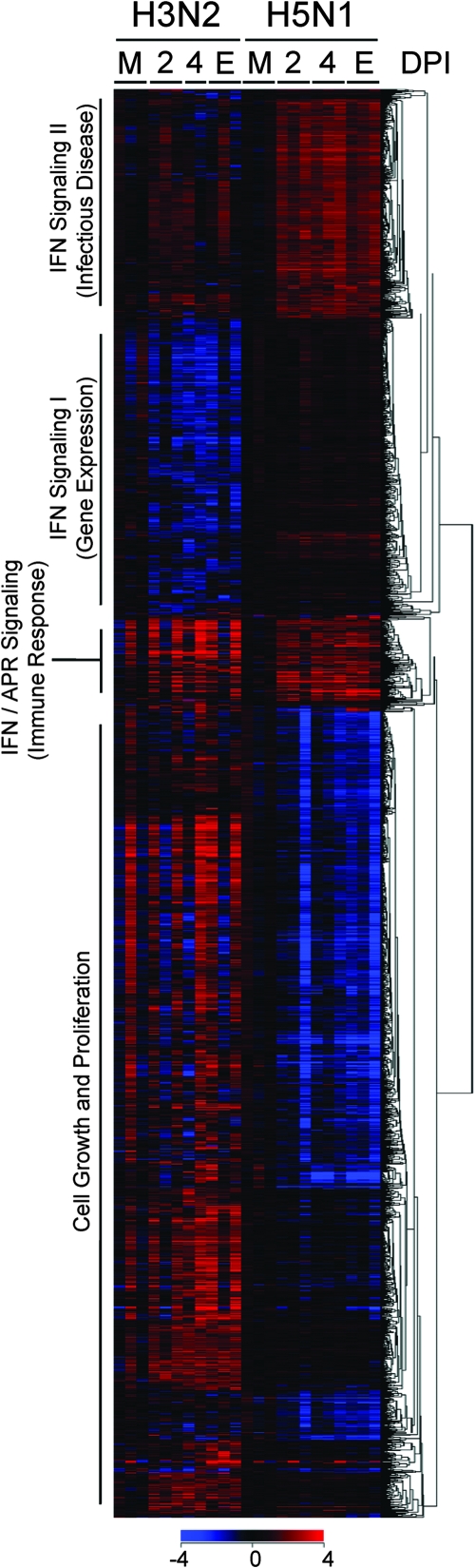
Our analysis strategy, which identified genes either up- or downregulated during H5N1 and H3N2 infection and differentially expressed between H5N1 and H3N2 infection, resulted in a list of 2,295 genes with significantly changed expression in lungs from influenza virus-infected ferrets. Hierarchical clustering analysis revealed several groups of coordinately expressed genes in four prominent functional clusters as defined by IPA, including a cell growth and proliferation gene network cluster and three unique gene clusters related to IFN signaling and innate immunity (Fig. 1). Genes in the cell growth and proliferation gene cluster were generally upregulated in the lungs of H3N2-infected ferrets and downregulated in the lungs of H5N1-infected ferrets. Genes in two of the IFN signaling clusters were generally upregulated in the lungs of H5N1-infected ferrets beginning at 2 dpi relative to their expression in H3N2-infected ferrets. A third IFN/acute-phase response signaling cluster, enriched in IFN and complement genes, was expressed similarly in both groups. A few ferrets appeared to behave differently in terms of gene expression from others in their group. The outbred status of the ferrets used in this study may be at least partially responsible for variation in individual host responses.
FIG. 1.
Four prominent gene expression clusters identified in H3N2- and H5N1-infected ferrets. Sequential Student's t tests (time point versus mock) identified genes significantly differentially expressed (P ≤ 0.05 and ≥2-fold change) for at least one time point and group during H3N2 and H5N1 infection in ferrets. These genes were combined with significantly differentially expressed genes (≥2-fold change for at least one time point and group and P ≤ 0.05) identified by EDGE analysis of differential gene expression between H3N2- and H5N1-infected ferrets. As shown, 2,295 significantly different genes were analyzed by one-way (by gene) hierarchical clustering (red, upregulated; blue, downregulated). The most significant signaling pathway(s) (IFN and IFN/acute-phase response signaling) or network (cell growth and proliferation, gene expression, immune response, and infectious disease) according to IPA for the resulting clusters are noted. M, mock. E, end point as described in Materials and Methods.
Table S2 in the supplemental material lists all four functional clusters and the genes included in each one of them. No one signaling pathway dominated the cell growth and proliferation gene network cluster; however, it was interesting to note that several genes associated with T- and B-cell signaling were significantly downregulated in H5N1-infected ferret lungs relative to H3N2-infected ferrets throughout the study period, namely, CD45 (CfaAffx.17631.1.S1_s_at; EDGE P = 0.046), GRB2 (CfaAffx.8081.1.S1_s_at; EDGE P = 0.010), and a number of phosphoinositide-3-kinase genes and mitogen-activated protein kinase genes (see Table S2 in the supplemental material). The primary aim of this study was to perform a detailed analysis on the three clusters heavily enriched in innate-immunity-related genes.
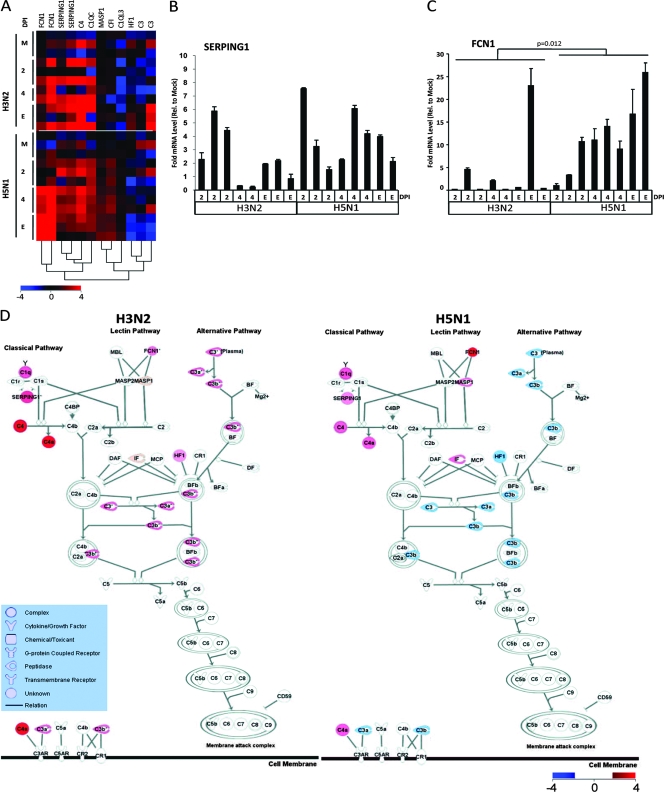
Complement is a critical component of innate immunity and appears to play a role in the host response to influenza virus infection (4, 5, 43); however, the extent of the involvement of the complement cascade in highly pathogenic influenza virus infection is not known. In our study, SERPING1, C4, and C1QC (see Table 1 for full gene names) were significantly upregulated at time points throughout H5N1 and H3N2 infection relative to in the mock-infected ferrets but were not differentially expressed between H5N1- and H3N2-infected ferrets; however, C1QL3, C3, HF1, MASP1, and FCN1 were significantly differentially expressed between H3N2- and H5N1-infected ferrets by EDGE analysis (Table 1 and Fig. 2A). C1q is a charge pattern recognition receptor which recognizes innate immune system targets and forms a complex with C1r and C1s to constitute the first component of the serum complement system, C1 (14). C1 complex activation is regulated by the serine protease inhibitor SERPING1 (incidentally also considered an IRG), which forms a proteolytically inactive stoichiometric complex with C1r, C1s, and MASP proteases (9). The similar upregulation of SERPING1 in H5N1- and H3N2-infected ferrets relative to the mock-infected ferrets was confirmed by qRT-PCR (Fig. 2B). C4 is cleaved by activated C1 to produce C4b, an essential component of the C3 and C5 convertases of the classical pathway. Ficolins are key mediators of innate immunity that trigger the lectin pathway of the complement cascade via activation of MASP zymogens, which in turn cleave the C4 component (13, 26). Ficolin 1 (FCN1) was expressed in both H3N2 and H5N1 infection at 2 to 4 dpi to the end point, but to a significantly greater degree in H5N1-infected ferrets (Table 1). The significantly higher expression of FCN1 in H5N1-infected ferret lungs was confirmed by qRT-PCR (Fig. 2C). Accordingly, the expression of one of the targets of FCN1, MASP1, was significantly upregulated in the lungs of H5N1-infected ferrets relative to H3N2-infected ferrets (Table 1). In a visual summary of the these results, Fig. 2D shows that while genes from the classical arm of the complement pathway were similarly upregulated in both groups of influenza virus-infected ferret lungs, FCN1 and MASP1 (lectin pathway) and C3 (common to all three complement pathways) were differentially regulated between H5N1- and H3N2-infected animals. C3 gene expression was significantly downregulated in the lungs of H5N1-infected ferrets at the end point relative to H3N2-infected ferrets (Fig. 2D and Table 1). Interestingly, C3-deficient mice have been shown to be highly susceptible to primary infection with influenza A virus and exhibit delayed viral clearance and increased viral titers in the lung (23). Moreover, HF1, an inhibitor of the alternative pathway, was upregulated only in H3N2-infected ferrets at the end point, suggestive of a negative feedback loop in the lungs of H3N2-infected ferrets. Taken together, our results suggest that complement activation and regulation may be important to the success of the transition from innate to specific antiviral immune responses during influenza A virus infection.
TABLE 1.
Expression of selected genes in H3N2 versus H5N1-infected ferrets
| Pathway and gene symbol | Name or description | Affymetrix identification | Entrez gene identificationa | Mean gene expression (H3N2/H5N1) atb:
|
EDGE P valuec | ||
|---|---|---|---|---|---|---|---|
| 2 dpi | 4 dpi | End point | |||||
| IFN signaling | |||||||
| CD274 | CD274 molecule | CfaAffx.4085.1.S1_at | 29126 | 0.73/0.19 | 0.6/0.58 | 0.79/1.93 | 0.036 |
| COPB2 | Coatomer protein complex, subunit beta 2 | Cfa.3255.1.A1_s_at | 9276 | −0.05/−0.29 | 1.04/0.69 | −0.17/0.21 | 0.890 |
| CSF2RA | Colony-stimulating factor 2 receptor, alpha | CfaAffx.17240.1.S1_at | 1438 | −0.2/−0.04 | −1.36/0.25 | −1.02/0.31 | 0.002 |
| CSNK1D | Casein kinase 1, delta | CfaAffx.10096.1.S1_s_at | 1453 | 0.63/1.35 | 0.37/1.62 | 0.62/1.48 | 0.053 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | Cfa.16590.1.S2_at | 3627 | 0.17/0.98 | 0.32/0.93 | −0.34/1.22 | 0.022 |
| FGG | Fibrinogen gamma chain | Cfa.5998.1.A1_x_at | 2266 | 0.65/−0.36 | 0.25/0.38 | 0.27/1.68 | 0.044 |
| FUBP1 | FUSE binding protein 1 | Cfa.19489.1.S1_at | 8880 | −0.58/0.18 | −1.27/0.08 | −0.95/0.34 | 0.030 |
| GEM | GTP binding protein overexpressed in skeletal muscle | CfaAffx.14457.1.S1_at | 2669 | −0.01/0.82 | −1.25/0.81 | −0.3/0.69 | 0.032 |
| GYS2 | Glycogen synthase 2 | Cfa.3601.1.S1_s_at | 2998 | −1.03/0.31 | −0.88/0.46 | 0.07/0.35 | 0.007 |
| HMMR | Hyaluronan-mediated motility receptor | CfaAffx.26316.1.S1_s_at | 3161 | −0.31/0.44 | −1.26/0.47 | −0.89/0.66 | <0.001 |
| IFI44 | IFN-induced protein 44 | CfaAffx.31148.1.S1_at | 10561 | 2.68/1.01 | 2.97/1.4 | 1.5/0.91 | 0.494 |
| IFI44L | IFN-induced protein 44-like | CfaAffx.31150.1.S1_at | 10964 | 1.57/0.41 | −0.21/1.24 | 0.08/0.92 | 0.201 |
| IFI6 | IFN-α-inducible protein 6 | Cfa.20456.1.S1_at | 2537 | 0.18/1.48 | −0.1/1.49 | −0.17/1.09 | 0.001 |
| IFITM1 | IFN-induced transmembrane protein 1 | CfaAffx.10684.1.S1_s_at | 10581 | 0.1/1.15 | 0.25/1.61 | 0.43/0.55 | 0.033 |
| IRF4 | IFN regulatory factor 4 | CfaAffx.11513.1.S1_at | 3662 | 0.25/1.09 | −0.44/1.55 | −0.42/1.17 | 0.009 |
| ISG15 | ISG15 ubiquitin-like modifier | Cfa.10757.1.S1_s_at | 9636 | 2.73/2.74 | 2.31/2.83 | 0.51/2.35 | 0.266 |
| JUN | Jun oncogene | CfaAffx.28854.1.S1_s_at | 3725 | 1.7/0.68 | 3.07/0.81 | 1.69/1.04 | 0.691 |
| MX2 | Myxovirus (influenza virus) resistance 2 | Cfa.3609.1.S1_s_at | 4600 | 0.55/0.75 | −0.04/1.11 | 0.16/0.83 | 0.193 |
| OAS1 | 2′,5′-Oligoadenylate synthetase 1 | Cfa.21191.1.S1_a_at | 4938 | 0.01/0.92 | 1.08/1.03 | 0.3/0.55 | 0.708 |
| OAS2 | 2′,5′-Oligoadenylate synthetase 2 | CfaAffx.14097.1.S1_s_at | 4939 | 1.09/0.81 | 1.36/1.15 | 0.51/1.08 | 0.433 |
| PBEF1 | Pre-B-cell colony-enhancing factor 1 | Cfa.18345.1.S1_s_at | 10135 | 0.42/−0.13 | 1.43/0.04 | 1.2/1.55 | 0.363 |
| PIAS1 | Protein inhibitor of activated STAT 1 | Cfa.19469.1.S1_s_at | 8554 | −0.72/−0.05 | −1.4/0.23 | −1.15/0.2 | 0.041 |
| PIM1 | Pim-1 oncogene | CfaAffx.3073.1.S1_at | 5292 | 0.37/0.92 | −0.84/1.09 | 0.34/0.83 | 0.110 |
| SERPING1 | Serpin peptidase inhibitor, clade G, 1 | Cfa.3117.2.A1_a_at | 710 | 1.73/1.34 | 1.11/1.63 | 2.15/0.95 | 0.526 |
| SERPING1 | Serpin peptidase inhibitor, clade G, 1 | CfaAffx.12560.1.S1_s_at | 710 | 0.97/0.98 | 0.51/1.62 | 1.48/0.79 | 0.640 |
| SOCS1 | Suppressor of cytokine signaling 1 | CfaAffx.28878.1.S1_at | 8651 | −0.32/0.59 | −1.65/0.48 | −0.34/0.7 | 0.048 |
| STAT1 | Signal transducer and activator of transcription 1 | CfaAffx.15419.1.S1_s_at | 6772 | 0.92/0.71 | 0.67/1.21 | 0.6/0.64 | 0.964 |
| STAT2 | Signal transducer and activator of transcription 2 | CfaAffx.1159.1.S1_at | 6773 | −0.8/−0.15 | −1.01/0.11 | −0.24/0.07 | 0.002 |
| TAP1 | Transporter 1 | Cfa.3155.1.S1_at | 6890 | 2.29/0.84 | 2.1/1.59 | 1.37/1.92 | 0.401 |
| UBE1L | Ubiquitin-activating enzyme E1-like | Cfa.3348.1.A1_s_at | 7318 | 1.15/2.05 | 1.11/2.47 | 0.99/1.87 | 0.401 |
| VAT1 | Vesicle amine transport protein 1 homolog | CfaAffx.22532.1.S1_s_at | 10493 | 0.36/1.02 | 0.09/1.08 | −0.4/0.87 | 0.007 |
| Complement | |||||||
| C1QC | Complement component 1, q subcomponent, C chain | Cfa.10921.1.S1_at | 714 | 0.81/1.71 | 2.36/1.58 | 1.47/1.25 | 0.848 |
| C1QL3 | Complement component 1, q subcomponent-like 3 | Cfa.9757.1.A1_at | 389941 | −1.3/0.28 | −1.3/0.28 | −1.07/0.49 | 0.040 |
| C3 | Complement component 3 | Cfa.12240.1.A1_at | 718 | −0.47/0.21 | −1.15/0.48 | 2.33/−1.6 | 0.004 |
| C3 | Complement component 3 | Cfa.13267.1.A1_s_at | 718 | −0.8/−0.17 | −1.37/−0.44 | 0.41/−1.07 | 0.020 |
| C4 | Complement component 4 | CfaAffx.1993.1.S1_s_at | 720 | 2.34/1.6 | 0.94/2.21 | 3.66/1.17 | 0.342 |
| CFI | Complement factor I (IF) | Cfa.14495.2.S1_at | 3426 | 0.48/1.15 | −0.46/1.25 | 0.06/1.12 | 0.084 |
| FCN1 | Ficolin 1 | Cfa.13207.1.A1_s_at | 2219 | 2.12/0.76 | 2.9/3.22 | 1.49/4.24 | 0.027 |
| FCN1 | Ficolin 1 | CfaAffx.30397.1.S1_at | 2219 | 0.8/0.21 | 1.33/2.24 | 0.92/3.11 | 0.001 |
| HF1 | Complement factor H | Cfa.20016.1.S1_at | 3075 | −0.46/−1.01 | −0.36/−0.14 | 0.9/−1.64 | 0.019 |
| MASP1 | Mannan-binding lectin serine peptidase 1 | CfaAffx.21390.1.S1_at | 5648 | 0.19/0.55 | 0.18/0.96 | 0.35/1.08 | 0.016 |
| SERPING1 | Serpin peptidase inhibitor, clade G, 1 | Cfa.3117.2.A1_a_at | 710 | 1.73/1.34 | 1.11/1.63 | 2.15/0.95 | 0.526 |
| SERPING1 | Serpin peptidase inhibitor, clade G, 1 | CfaAffx.12560.1.S1_s_at | 710 | 0.97/0.98 | 0.51/1.62 | 1.48/0.79 | 0.640 |
The Entrez gene identification corresponds to the human homolog. E, endpoint as described in Materials and Methods.
Mean gene expression is normalized to the means for the corresponding mock infection (log2 ratio). Boldface indicates significantly differentially expressed genes (P ≤ 0.05) versus the mock infection by the sequential Student's t tests.
Statistical significance of gene expression differences between H3N2- and H5N1-infected ferrets determined by the EDGE analysis.
FIG. 2.
Complement cascade activation in the lungs of influenza virus-infected ferrets. (A) One-way hierarchical clustering of complement-related genes selected by IPA from the three innate-immunity-related gene clusters in Fig. 1 (red, upregulated; blue, downregulated). (B) qRT-PCR analysis of SERPING1 gene expression performed in triplicate. Error bars indicate standard deviations. (C) qRT-PCR analysis of FCN1 performed in triplicate. Error bars indicate standard deviations, and the Mann-Whitney P value is shown. (D) IPA canonical pathway modeling of the complement system using the microarray analysis of gene expression data from the lungs of influenza virus-infected ferrets at the end point (red, upregulated; blue, downregulated). M, mock. E, end point as described in Materials and Methods.
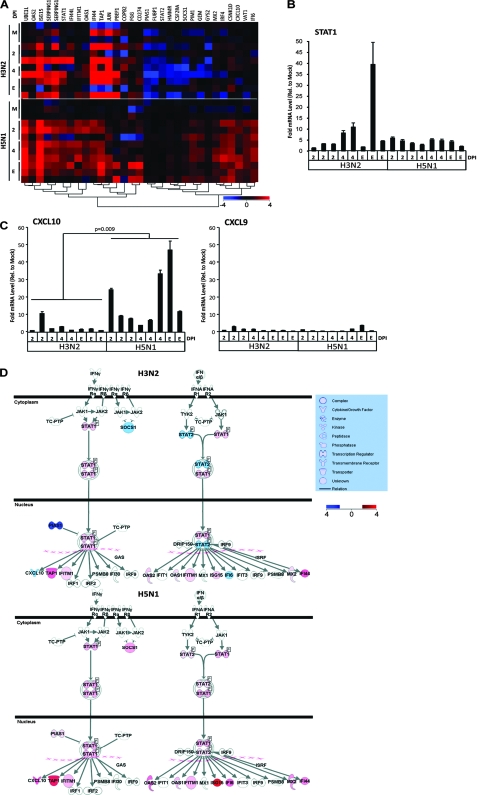
We next looked at the expression of the large variety of IRGs identified in the three innate immunity-related clusters shown in Fig. 1. IRGs are a large family of IFN-signaling and IFN-stimulated immune mediators with pleiotropic downstream functions in innate antiviral responses and host defense (34). Figure 3A and Table 1 show that many IRGs, such as IFI44, ISG15 (G1P2), MX2, OAS1, OAS2, STAT1, TAP1, and UBE1L, were significantly upregulated throughout the study period in both H5N1- and H3N2-infected ferret lungs relative to the mock-infected ferret lungs. These genes, while coordinately expressed, have diverse functions in host defense. IFI44, MX2, and OAS2 are antiviral IRGs induced mainly by type I IFNs (IFN-α and IFN-β), while OAS1 is induced by type I and II (IFN-γ) IFNs and mediates cellular resistance against viral infection (UNIPROT). UBE1L catalyzes the initial step of ISG15 activation, a target of influenza NS1B virus inhibition (41). ISG15 is an important ubiquitin-like modifier that conjugates with critical intracellular antiviral targets upon type I IFN stimulation, such as JAK1 and STAT1 (27). STAT1 is phosphorylated by Jak kinases upon type I and II IFN ligation of their cognate cell surface receptors, leading to IRG transcription and initiation of an antiviral cellular state (25). The similar upregulation of STAT1 in H5N1- and H3N2-infected ferrets relative to the mock-infected ferrets was confirmed by qRT-PCR (Fig. 3B). TAP1, induced by IFN-γ, is critical for major histocompatibility complex class I antigen presentation, thereby facilitating the identification and eradication of virally infected cells (1).
FIG. 3.
IFN signaling pathway activation in the lungs of influenza virus-infected ferrets. (A) One-way hierarchical clustering of IRGs selected by IPA from the three innate immunity-related gene clusters in Fig. 1 (red, upregulated; blue, downregulated). (B) qRT-PCR analysis of STAT1 performed in triplicate. Error bars indicate standard deviations. (C) Left, qRT-PCR analysis of CXCL10. The Mann-Whitney P value is shown. Right, qRT-PCR analysis of CXCL9. Analyses were performed in triplicate, and error bars indicate standard deviations. (D) IPA modeling of the IFN signaling pathway using the microarray analysis of gene expression data from the lungs of influenza virus-infected ferrets at the end point (red, upregulated; blue, downregulated). M, mock. E, end point as described in Materials and Methods.
Significant differences in the expression of other IRGs between H3N2- and H5N1-infected ferret lungs occurred throughout course of disease (Table 1). STAT2 gene expression was significantly downregulated in H3N2-infected ferrets versus H5N1-infected ferrets (Fig. 3A and Table 1). As modeled in IPA at the end point in Fig. 3D, increases in STAT2 transcription upon type I IFN stimulation may result in increased levels of STAT2 protein available for dimerization with STAT1 and serve to increase the transcription of many antiviral IRGs (34). Indeed, significant upregulation of CD274, IFI6 (G1P3), IFITM1, and IRF4 was noted in H5N1- versus H3N2-infected ferret lungs at time points throughout the course of disease (Table 1). CD274 (B7-H1) is upregulated on lymphocytes upon IFN-γ activation and plays a role in T-cell costimulation (UNIPROT) and apoptosis during viral infections (28). IFI6 is an antiviral response protein induced by type I IFNs with a particular potency in suppressing hepatitis C virus (17). IFITM1 is induced by type I and II IFNs and is involved in the control of cell growth and proliferation (10). Interestingly, IRF4 is not induced by IFNs but binds to the IFN-stimulated response element of the major histocompatibility complex class I promoter (UNIPROT) and has been implicated in the control of T-helper 2 (Th2) cell subset differentiation (36). SOCS1, a negative feedback regulator of cytokine signaling, was significantly downregulated in H3N2-infected ferrets and increased in the H5N1-infected ferrets (Table 1 and Fig. 3D). Likewise, PIAS1, a direct inhibitor of STAT1, is significantly attenuated in H3N2-infected ferrets and upregulated in H5N1-infected ferrets (Table 1 and Fig. 3D). Overall, our microarray analysis indicates that H5N1 and H3N2 influenza A virus infections result in notable IFN-mediated antiviral host responses in the lungs of ferrets. However, hyperinduction and persistent expression of certain IRGs in the lungs of H5N1-infected ferrets, despite functional negative feedback and perhaps in conjunction with suppressed T- and B-cell signaling (Fig. 1), may provoke the immune dysregulation characteristic of highly pathogenic H5N1 influenza virus.
CXCL10 gene expression was significantly upregulated in H5N1-infected ferret lungs throughout the course of the study relative to H3N2-infected ferret lungs as confirmed by qRT-PCR and EDGE analysis (Fig. 3C and Table 1). CXCL10 is a potent chemoattractant for activated Th1 lymphocytes and natural killer cells and is thought to play a role in the temporal development of innate and adaptive immunity in concert with type I and II IFNs (29). CXCL10's cognate receptor is CXCR3, which also binds CXCL9 and CXCL11. The expression of CXCL9 and CXCL11 could not be measured by microarray due to the absence of the probes on the Canine 2.0 array; however, by qRT-PCR we showed that CXCL9 expression was not induced to the level of CXCL10 expression during influenza virus infection and was not significantly different between H5N1- and H3N2-infected ferret lungs relative to the mock infection (Fig. 3C). The drug AMG487 is a potent antagonist of CXCR3 and can inhibit cell migration mediated by CXCL9, CXCL10, and CXCL11, thereby blocking cellular recruitment (19) and tumor metastasis (38) in mice. We therefore tested whether attenuation of CXCR3 signaling by AMG487 could alter the disease course in H5N1-infected ferrets. We challenged 17 ferrets with H5N1 virus and randomly assigned 8 to treatment with AMG487 and 9 to treatment with PBS as per the schedule described in Materials and Methods. AMG487 treatment significantly abrogated hypothermia (Fig. 4a), increased SpO2 levels (Fig. 4b), decreased weight loss (Fig. 4c), and improved daily activity scores (Fig. 4d). AMG487 treatment also resulted in a significant shift in the length of survival (Fig. 4e) in that all vehicle-treated ferrets were deceased by the end of 6 dpi while 25% of the AMG487-treated ferrets survived until the end of 7 or 8 dpi. Nasal wash viral loads were not significantly different between AMG487- and vehicle-treated ferrets (data not shown); however, AMG487-treated ferrets exhibited significantly reduced viral loads in the lungs compared to controls at 6 dpi (Fig. 4f). Accordingly, AMG487-treated ferret lungs showed a marked reduction in interstitial and alveolar edema and infiltrate compared with controls at 6 dpi (Fig. 4g and h). Therefore, even though our model of infection of ferrets with H5N1 influenza virus at 1 × 106 EID50 showed 100% lethality, blockade of the CXCL9, CXCL10, and CXCL11-CXCR3 signaling axis by AMG487 treatment caused a significant shift in the kinetics of viral replication in the lung and in the clinical course of disease.
FIG. 4.
AMG487 treatment improves respiratory function and delays mortality in H5N1-infected ferrets. Ferrets were infected with H5N1 as described in Materials and Methods and received either 1.65 mg/kg AMG487 in a 3-ml volume of PBS (n = 8) or PBS vehicle (n = 9) intraperitoneally every 12 h starting at 24 h postinfection and continuing until the end point. Weight loss, activity, temperature, and SpO2 (pulse oximetry) were monitored daily from 2 dpi. (a) Change in body temperature relative to baseline means (P ≤ 0.001 by two-way ANOVA). (b) SpO2 (P ≤ 0.005 by two-way ANOVA). (c) Weight loss (percentage of original body mass; P ≤ 0.001 by two-way ANOVA). (d) Mean daily activity scores: 0, normal; 1, alert, playful when stimulated; 2, alert, not playful when stimulated; 3, neither alert nor playful when stimulated (P ≤ 0.05 by two-way ANOVA). (e) Kaplan-Meier survival curves for AMG487 (Exp.)- versus vehicle (Control)-treated ferrets. Curves are significantly different (P ≤ 0.05) by log rank analysis. (f) Lung viral titers in three ferrets from each treatment group euthanized at 6 dpi (*, P ≤ 0.05 by Student's t test). (g and h) Representative hematoxylin- and eosin-stained lung sections at 6 dpi (magnification, ×4). Error bars represent standard errors of the means in all panels.
Vaccines are the ideal means of protecting the population against an influenza outbreak, and a number of promising candidates have been recently tested (2, 16, 42). Unfortunately, influenza virus vaccine efficacy is variable in certain populations, such as the elderly (18), and the emergence of novel strains of influenza virus for which vaccines are not immediately available requires the development of other strategies. While antivirals may prove beneficial in the treatment of avian influenza virus disease in humans, circulating strains of H5N1 influenza virus exhibit variable susceptibility to antiviral agents, with full resistance to M2 inhibitors displayed by clade 1 and most clade 2 viruses and high levels of resistance to oseltamivir in clade 2 viruses. Ideally, a treatment which does not rely on virus strain specificity and one that does not allow for the development of virus resistance would complement vaccine and antiviral strategies. AMG487 has shown clinical promise in animal models and has been well tolerated in human phase II clinical trials (reviewed in reference 39), but its clinical potential in treating influenza is unknown. With appropriate future study, AMG487 treatment may demonstrate effectiveness in combination with other strategies, such as antiviral treatment, in reducing lung immunopathology, establishing effective antiviral IRG responses, and reducing viral burden during H5N1 infection.
One caveat of our study is that differences between H5N1 and H3N2 pathogenicity in the lung during infection may not have been directly reflected by the microarray analysis, since H3N2 did not replicate to detectable levels in the ferret lungs. H3N2 infection served as an important background against which to identify potential host responses specific to H5N1 infection in ferrets for further analysis. Also, the lack of availability of ferret-specific reagents and sequences limited the extent to which we could validate our microarray results by qRT-PCR and/or protein assays. Nonetheless, our results are significant in that we have identified innate immune genes that are similarly expressed during H5N1 and H3N2 infection, as well as notable genes involved in complement and IFN signaling that are differentially expressed in the respiratory tracts of H5N1 influenza virus-infected ferrets and H3N2-infected ferrets.
Several recent microarray studies have highlighted the common involvement of IFN responses in the acute phase of uncomplicated influenza virus infections in humans, macaques, and rodents (3, 21, 30); however, insufficient knowledge exists regarding the mechanisms of host inflammatory cytokine and chemokine responses in severe H5N1 pathogenesis (11, 32, 33, 40). Mechanistically, we have attenuated the action of CXCL10 by blocking its activation of CXCR3 using AMG487 drug treatment, thereby reducing pulmonary viral load and pathology, improving respiratory function, and eliciting a modest yet statistically significant delay in mortality in H5N1-infected ferrets. Previous in vitro results show that H5N1-infected primary human alveolar and bronchial epithelial cells have a greater capacity for CXCL10 induction than those infected with common strains of human influenza virus (8). In agreement with our current results, it has also been shown that CXCL10 and not CXCL9 expression correlates with pharyngeal viral load in human H5N1 infections (11). Likewise, we have previously shown that high levels of CXCL10 and not CXCL9 are associated with persistent severe viral disease in patients with severe acute respiratory syndrome (6, 7). Finally, the severity and outcome of 1918 influenza virus infection in a macaque model may be determined by dysregulated IFN responses that arise during host innate immunity (22). Our results provide further evidence that IRGs, in particular CXCL10, may have pathological importance in H5N1 infection and are at least partially responsible for disease pathogenesis. The parallels among the immunopathologies of severe acute respiratory syndrome virus, 1918 influenza virus, and avian influenza virus (H5N1) suggest a common underlying mechanism in the natural disease course of these infections. In this regard, AMG487 treatment has unique possibilities in correcting dysregulated host responses during severe respiratory viral illnesses and warrants further exploration in complementing existing potential therapies.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research, the Li Ka Shing Foundation, and the National Institutes of Health.
C.M.C. designed and carried out experiments, analyzed data, and wrote the manuscript. M.J.C. performed genomics analysis and wrote the manuscript. J.F.B.-M. provided experimental and clinical design. L.R. and L.X. carried out microarray data and sample processing. P.V.T. analyzed histochemistry. A.D., R.R., Y.F., and P.-K.M.C. designed qRT-PCR primers or methodology. N.M. carried out animal procedures. T.J.S., T.L.C., M.G.J., and J.C.M. supplied AMG487 and advised on its use. T.R. performed animal experiments and wrote the manuscript. D.J.K. provided laboratory and financial resources, designed experiments, and wrote the manuscript.
T.J.S., T.L.C., M.G.J., and J.C.M. are employees of Amgen Inc.
Footnotes
Published ahead of print on 6 August 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abele, R., and R. Tampe. 2004. The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology (Bethesda) 19216-224. [DOI] [PubMed] [Google Scholar]
- 2.Baras, B., K. J. Stittelaar, J. H. Simon, R. J. Thoolen, S. P. Mossman, F. H. Pistoor, G. van Amerongen, M. A. Wettendorff, E. Hanon, and A. D. Osterhaus. 2008. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One 3e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baskin, C. R., A. Garcia-Sastre, T. M. Tumpey, H. Bielefeldt-Ohmann, V. S. Carter, E. Nistal-Villan, and M. G. Katze. 2004. Integration of clinical data, pathology, and cDNA microarrays in influenza virus-infected pigtailed macaques (Macaca nemestrina). J. Virol. 7810420-10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beebe, D. P., R. D. Schreiber, and N. R. Cooper. 1983. Neutralization of influenza virus by normal human sera: mechanisms involving antibody and complement. J. Immunol. 1301317-1322. [PubMed] [Google Scholar]
- 5.Bjornson, A. B., M. A. Mellencamp, and G. M. Schiff. 1991. Complement is activated in the upper respiratory tract during influenza virus infection. Am. Rev. Respir. Dis. 1431062-1066. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, M. J., J. F. Bermejo-Martin, A. Danesh, M. P. Muller, and D. J. Kelvin. 2008. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 13313-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron, M. J., L. Ran, L. Xu, A. Danesh, J. F. Bermejo-Martin, C. M. Cameron, M. P. Muller, W. L. Gold, S. E. Richardson, S. M. Poutanen, B. M. Willey, M. E. Devries, Y. Fang, C. Seneviratne, S. E. Bosinger, D. Persad, P. Wilkinson, L. D. Greller, R. Somogyi, A. Humar, S. Keshavjee, M. Louie, M. B. Loeb, J. Brunton, A. J. McGeer, and D. J. Kelvin. 2007. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 818692-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, M. C., C. Y. Cheung, W. H. Chui, S. W. Tsao, J. M. Nicholls, Y. O. Chan, R. W. Chan, H. T. Long, L. L. Poon, Y. Guan, and J. S. Peiris. 2005. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicardi, M., L. Zingale, A. Zanichelli, E. Pappalardo, and B. Cicardi. 2005. C1 inhibitor: molecular and clinical aspects. Springer Semin. Immunopathol. 27286-298. [DOI] [PubMed] [Google Scholar]
- 10.Deblandre, G. A., O. P. Marinx, S. S. Evans, S. Majjaj, O. Leo, D. Caput, G. A. Huez, and M. G. Wathelet. 1995. Expression cloning of an interferon-inducible 17-kDa membrane protein implicated in the control of cell growth. J. Biol. Chem. 27023860-23866. [DOI] [PubMed] [Google Scholar]
- 11.de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, D. Q. Ha, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 121203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Veer, M. J., M. Holko, M. Frevel, E. Walker, S. Der, J. M. Paranjape, R. H. Silverman, and B. R. Williams. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69912-920. [PubMed] [Google Scholar]
- 13.Frederiksen, P. D., S. Thiel, C. B. Larsen, and J. C. Jensenius. 2005. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand. J. Immunol. 62462-473. [DOI] [PubMed] [Google Scholar]
- 14.Ghai, R., P. Waters, L. T. Roumenina, M. Gadjeva, M. S. Kojouharova, K. B. Reid, R. B. Sim, and U. Kishore. 2007. C1q and its growing family. Immunobiology 212253-266. [DOI] [PubMed] [Google Scholar]
- 15.Govorkova, E. A., J. E. Rehg, S. Krauss, H. L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffman. 2006. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 806195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh, Y., H. Ozaki, H. Tsuchiya, K. Okamoto, R. Torii, Y. Sakoda, Y. Kawaoka, K. Ogasawara, and H. Kida. 2008. A vaccine prepared from a non-pathogenic H5N1 avian influenza virus strain confers protective immunity against highly pathogenic avian influenza virus infection in cynomolgus macaques. Vaccine 26562-572. [DOI] [PubMed] [Google Scholar]
- 17.Itsui, Y., N. Sakamoto, M. Kurosaki, N. Kanazawa, Y. Tanabe, T. Koyama, Y. Takeda, M. Nakagawa, S. Kakinuma, Y. Sekine, S. Maekawa, N. Enomoto, and M. Watanabe. 2006. Expressional screening of interferon-stimulated genes for antiviral activity against hepatitis C virus replication. J. Viral Hepat. 13690-700. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson, T., D. Rivetti, A. Rivetti, M. Rudin, C. Di Pietrantonj, and V. Demicheli. 2005. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 3661165-1174. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, M., A. R. Li, J. Liu, Z. Fu, L. Zhu, S. Miao, X. Wang, Q. Xu, A. Huang, A. Marcus, F. Xu, K. Ebsworth, E. Sablan, J. Danao, J. Kumer, D. Dairaghi, C. Lawrence, T. Sullivan, G. Tonn, T. Schall, T. Collins, and J. Medina. 2007. Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg. Med. Chem. Lett. 173339-3343. [DOI] [PubMed] [Google Scholar]
- 20.Kandun, I. N., H. Wibisono, E. R. Sedyaningsih, Yusharmen, W. Hadisoedarsuno, W. Purba, H. Santoso, C. Septiawati, E. Tresnaningsih, B. Heriyanto, D. Yuwono, S. Harun, S. Soeroso, S. Giriputra, P. J. Blair, A. Jeremijenko, H. Kosasih, S. D. Putnam, G. Samaan, M. Silitonga, K. H. Chan, L. L. Poon, W. Lim, A. Klimov, S. Lindstrom, Y. Guan, R. Donis, J. Katz, N. Cox, M. Peiris, and T. M. Uyeki. 2006. Three Indonesian clusters of H5N1 virus infection in 2005. N. Engl. J. Med. 3552186-2194. [DOI] [PubMed] [Google Scholar]
- 21.Kawada, J., H. Kimura, Y. Kamachi, K. Nishikawa, M. Taniguchi, K. Nagaoka, H. Kurahashi, S. Kojima, and T. Morishima. 2006. Analysis of gene-expression profiles by oligonucleotide microarray in children with influenza. J. Gen. Virol. 871677-1683. [DOI] [PubMed] [Google Scholar]
- 22.Kobasa, D., S. M. Jones, K. Shinya, J. C. Kash, J. Copps, H. Ebihara, Y. Hatta, J. H. Kim, P. Halfmann, M. Hatta, F. Feldmann, J. B. Alimonti, L. Fernando, Y. Li, M. G. Katze, H. Feldmann, and Y. Kawaoka. 2007. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445319-323. [DOI] [PubMed] [Google Scholar]
- 23.Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M. F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8373-378. [DOI] [PubMed] [Google Scholar]
- 24.Leek, J. T., E. Monsen, A. R. Dabney, and J. D. Storey. 2006. EDGE: extraction and analysis of differential gene expression. Bioinformatics 22507-508. [DOI] [PubMed] [Google Scholar]
- 25.Leonard, W. J., and J. J. O'Shea. 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16293-322. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y., Y. Endo, D. Iwaki, M. Nakata, M. Matsushita, I. Wada, K. Inoue, M. Munakata, and T. Fujita. 2005. Human M-ficolin is a secretory protein that activates the lectin complement pathway. J. Immunol. 1753150-3156. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y. C., J. Penninger, and M. Karin. 2005. Immunity by ubiquitylation: a reversible process of modification. Nat. Rev. Immunol. 5941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlbauer, M., M. Fleck, C. Schutz, T. Weiss, M. Froh, C. Blank, J. Scholmerich, and C. Hellerbrand. 2006. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J. Hepatol. 45520-528. [DOI] [PubMed] [Google Scholar]
- 29.Neville, L. F., G. Mathiak, and O. Bagasra. 1997. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 8207-219. [DOI] [PubMed] [Google Scholar]
- 30.Pennings, J. L., T. G. Kimman, and R. Janssen. 2008. Identification of a common gene expression response in different lung inflammatory diseases in rodents and macaques. PLoS One 3e2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomon, R., J. Franks, E. A. Govorkova, N. A. Ilyushina, H. L. Yen, D. J. Hulse-Post, J. Humberd, M. Trichet, J. E. Rehg, R. J. Webby, R. G. Webster, and E. Hoffmann. 2006. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 203689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salomon, R., E. Hoffmann, and R. G. Webster. 2007. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. 10412479-12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szretter, K. J., S. Gangappa, X. Lu, C. Smith, W. J. Shieh, S. R. Zaki, S. Sambhara, T. M. Tumpey, and J. M. Katz. 2007. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 812736-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaoka, A., and H. Yanai. 2006. Interferon signalling network in innate defence. Cell. Microbiol. 8907-922. [DOI] [PubMed] [Google Scholar]
- 35.To, K. F., P. K. Chan, K. F. Chan, W. K. Lee, W. Y. Lam, K. F. Wong, N. L. Tang, D. N. Tsang, R. Y. Sung, T. A. Buckley, J. S. Tam, and A. F. Cheng. 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 63242-246. [DOI] [PubMed] [Google Scholar]
- 36.Tominaga, N., K. Ohkusu-Tsukada, H. Udono, R. Abe, T. Matsuyama, and K. Yui. 2003. Development of Th1 and not Th2 immune responses in mice lacking IFN-regulatory factor-4. Int. Immunol. 151-10. [DOI] [PubMed] [Google Scholar]
- 37.Ungchusak, K., P. Auewarakul, S. F. Dowell, R. Kitphati, W. Auwanit, P. Puthavathana, M. Uiprasertkul, K. Boonnak, C. Pittayawonganon, N. J. Cox, S. R. Zaki, P. Thawatsupha, M. Chittaganpitch, R. Khontong, J. M. Simmerman, and S. Chunsutthiwat. 2005. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 352333-340. [DOI] [PubMed] [Google Scholar]
- 38.Walser, T. C., S. Rifat, X. Ma, N. Kundu, C. Ward, O. Goloubeva, M. G. Johnson, J. C. Medina, T. L. Collins, and A. M. Fulton. 2006. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 667701-7707. [DOI] [PubMed] [Google Scholar]
- 39.Wijtmans, M., D. Verzijl, R. Leurs, I. J. de Esch, and M. J. Smit. 2008. Towards small-molecule CXCR3 ligands with clinical potential. Chem. Med. Chem. 3861-872. [DOI] [PubMed] [Google Scholar]
- 40.Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358261-273. [DOI] [PubMed] [Google Scholar]
- 41.Yuan, W., and R. M. Krug. 2001. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 20362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zangwill, K. M., J. J. Treanor, J. D. Campbell, D. L. Noah, and J. Ryea. 2008. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J. Infect. Dis. [DOI] [PubMed]
- 43.Zhang, H., Y. A. Su, P. Hu, J. Yang, B. Zheng, P. Wu, J. Peng, Y. Tang, and L. Zhang. 2006. Signature patterns revealed by microarray analyses of mice infected with influenza virus A and Streptococcus pneumoniae. Microbes. Infect. 82172-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 764420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.