Abstract
Isoleucine deprivation of cellular monolayers prior to infection has been reported to result in partial complementation of a herpes simplex virus type 1 (HSV-1) ICP0 null (ICP0−) mutant. We now report that glutamine deprivation alone is able to enhance the plating efficiency of an ICP0− virus and that isoleucine deprivation has little or no effect. Because a low glutamine level is associated with stress and because stress is known to induce reactivation, low levels of glutamine may be relevant to the reactivation of HSV-1 from latency. Additionally, we demonstrate that arginine and methionine deprivation result in partial complementation of the ICP0− virus.
The immediate-early protein ICP0 of herpes simplex virus type 1 (HSV-1) plays major roles in lytic infection and reactivation from latency. During lytic infection, ICP0 functions as a potent and broad transcriptional activator (6, 10, 13, 19, 20), though it does not bind DNA in solution (11). ICP0 also plays important roles in the establishment of latency (9, 32) and reactivation from latency (15, 16, 29). A further level of ICP0 complexity was reported by Cai and Schaffer, who demonstrated that 8 h after release of an isoleucine (Ile) block to synchronize cells in G0/G1 phase (22, 30), cells began cycling out of G1 phase and into S phase, and the peak plating efficiency of the ICP0 null (ICP0−) virus occurred during this transition (7). The conclusion from this experiment was that ICP0− viruses form plaques more efficiently in late G1/early S phase than in other phases of the cell cycle. We now believe that this conclusion is incorrect.
In an earlier publication, we showed that the plating efficiency of an ICP0− virus is enhanced following Ile deprivation or serum starvation, but only in the absence of glutamine (Gln) (5). In the presence of Gln, Ile deprivation and serum starvation result in synchrony of cells in G0/G1 phase, but the plating efficiency of an ICP0− virus is not enhanced at any stage of the cell cycle. We have considered the following two potential explanations for these observations: (i) Gln prevents the enhanced plating efficiency that results from Ile deprivation or serum starvation or (ii) Gln deprivation causes the enhanced plating efficiency. We present evidence herein that the second hypothesis is correct.
Gln deprivation, but not Ile deprivation, enhances ICP0− virus plating efficiency.
Twenty-four-hour-old Vero cell monolayers were incubated for 72 h in media supplemented with 10% dialyzed fetal bovine serum and containing Ile and Gln or lacking one or both amino acids. Dulbecco's modified Eagle's medium (DMEM) containing both amino acids and normal fetal bovine serum was re-added for 8 h (to obtain peak plating efficiency following the release of an Ile block) (5, 7), and monolayers were infected with wild-type or ICP0− virus.
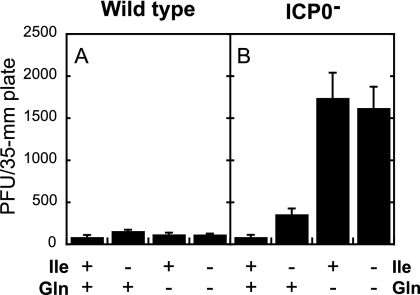
The presence or absence of Ile and Gln had little effect on the plating efficiency of the wild-type virus (Fig. 1A). The plating efficiency of the ICP0− virus was greatly enhanced (18-fold) when Ile and Gln were both absent or when only Gln was absent (20-fold) relative to that in the presence of both amino acids (Fig. 1B). In contrast, the plating efficiency of the ICP0− virus was much lower following Ile deprivation in the presence of Gln (fourfold enhancement over that in the presence of Ile and Gln). These observations demonstrate that the total enhancement in plating efficiency of an ICP0− virus can be attributed specifically and exclusively to Gln deprivation.
FIG. 1.
The plating efficiency of an ICP0− virus is enhanced when cells are incubated in the absence of Gln but not the absence of Ile. Twenty-four-hour-old Vero cells were incubated for 72 h in media containing Ile and Gln or lacking only Ile, only Gln, or both. Monolayers were infected with 10 to 100 PFU/plate wild-type (KOS) (A) or ICP0− (n212) (B) virus in complete DMEM. After a 1-h infection, monolayers were overlaid with complete DMEM containing methyl cellulose and incubated for 5 days at 37°C. Plaques were counted and multiplied by a dilution factor of 1 to 10 to normalize all plaque counts to infection with 100 PFU/plate. Bars represent the average numbers of PFU/plate in three independent experiments, and error bars represent the standard deviations.
Note for this and subsequent experiments that the ICP0− virus does not plate more efficiently than the wild-type virus, even following Gln deprivation. ICP0− viruses form plaques 16- to 50-fold less efficiently than wild-type virus on Vero cells (7, 8, 27, 35), so a higher input was required for the ICP0 mutant.
Gln deprivation also resulted in enhanced plating efficiency of the ICP0 mutant dl1403 but not the parental wild-type strain 17 (data not shown). This result demonstrates that enhanced plating efficiency following Gln deprivation is not restricted to the KOS derivative n212.
In light of these findings, how do we explain the results in Fig. 1 of the Cai and Schaffer publication (7)? It was reported that 2 mM Gln was present in the Ile-free medium, but we have now demonstrated that the plating efficiency of an ICP0− virus is not enhanced in the presence of 2 mM Gln. We believe that the experimental medium used in the Cai and Schaffer experiments initially contained 2 mM Gln but that, over time, the amount of Gln fell to a very low concentration due to the instability of Gln in solution (17, 21, 31).
In addition to Gln deprivation, arginine (Arg) and methionine (Met) deprivation also causes enhanced plating efficiency of an ICP0− virus.
As noted above, depriving cells of Gln results in enhanced plating efficiency of an ICP0− virus. We were interested to know if depriving cells of other amino acids would affect the plating efficiency of an ICP0− virus.
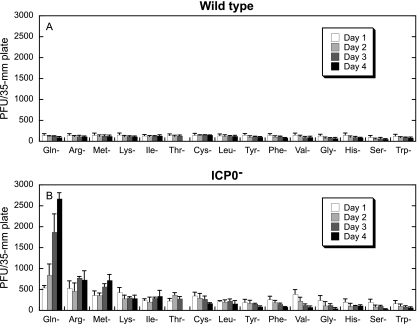
To test this possibility, 24-h-old Vero cell monolayers were incubated for 1 to 4 days in media lacking a single amino acid. All 15 amino acids included in DMEM were tested. Each day, a monolayer incubated in each type of medium was refed with complete medium for 8 h and infected with wild-type or ICP0− virus.
The plating efficiency of the wild-type virus was not affected by the absence of any of the amino acids (Fig. 2A). However, for most amino acids, the plating efficiency of the wild-type virus decreased over time as cells died due to amino acid deprivation, and the density of the monolayers decreased. For the ICP0− virus, deprivation of Gln, Arg, or Met resulted in the greatest number of plaques (Fig. 2B). For these three amino acids, the plating efficiency increased with an increasing duration of amino acid deprivation. For the remaining amino acids, a measurable decrease in plating efficiency was evident with increasing time of deprivation, or no change was observed. The decrease in plating efficiency corresponded with cell death due to amino acid deprivation and was observed as decreased cell densities of the monolayers.
FIG. 2.
Deprivation of Gln, Arg, or Met results in enhanced ICP0− virus plating efficiency. Twenty-four-hour-old Vero cell monolayers were incubated in media lacking 1 of the 15 amino acids included in the DMEM formulation. Each day for 4 days, monolayers were infected with 5 to 100 PFU of wild-type (KOS) (A) or ICP0− (n212) (B) virus in complete DMEM. After a 1-h infection, monolayers were overlaid with complete DMEM containing methyl cellulose and incubated for 5 days at 37°C. Plaques were counted and multiplied by a dilution factor of 1 to 20 to normalize all plaque counts to infection with 100 PFU/plate. Bars represent the average numbers of PFU/plate in three independent experiments, and error bars represent the standard deviations.
The simultaneous absence of both Arg and Met increases the plating efficiency of an ICP0− virus to the same extent as that by Gln deprivation.
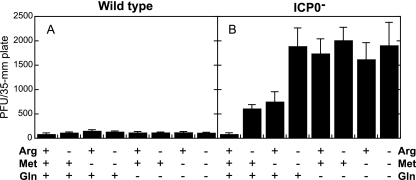
Having shown that Arg or Met deprivation can enhance the plating efficiency of an ICP0− virus, we were interested to know if deprivation of both amino acids in combination would further increase the plating efficiency of the virus.
To test this possibility, 24-h-old Vero cell monolayers were incubated for 72 h in the presence of all amino acids or in the absence of Arg, Met, Gln, or combinations of these three amino acids. Media were replaced with complete DMEM for 8 h, and monolayers were infected with wild-type or ICP0− virus (Fig. 3). The plating efficiency of the wild-type virus was not affected by deprivation of any of these three amino acids, individually or in combination (Fig. 3A). The ICP0− virus exhibited sevenfold and eightfold enhanced plating efficiencies following deprivation of Arg or Met, respectively, relative to the plating efficiency on monolayers incubated with all three amino acids (Fig. 3B). Gln deprivation resulted in a 20-fold enhanced plating efficiency relative to that in medium with all amino acids. However, when cells were deprived of both Arg and Met simultaneously, a synergistic effect resulting in a plating efficiency similar to that with Gln deprivation was noted. In contrast, deprivation of Gln and Arg, Gln and Met, or all three amino acids did not enhance the plating efficiency relative to that with Gln deprivation alone.
FIG. 3.
Deprivation of Arg and Met results in enhanced plating efficiency of an ICP0− virus to a similar extent to that with Gln deprivation. Twenty-four-hour-old Vero cells were incubated for 72 h in media containing Arg, Met, and Gln or lacking one, two, or all of these amino acids. Monolayers were infected with 100 PFU wild-type (KOS) (A) or ICP0− (n212) (B) virus in complete DMEM. After a 1-h infection, monolayers were overlaid with complete DMEM containing methyl cellulose and incubated for 5 days at 37°C. Plaques were counted and multiplied by a dilution factor of 1 to 20 to normalize all plaque counts to infection with 100 PFU/plate. Bars represent the average numbers of PFU/plate in three independent experiments, and error bars represent the standard deviations.
Enhanced plating efficiency of ICP0− viruses following Gln deprivation is not due to a decrease in cell density.
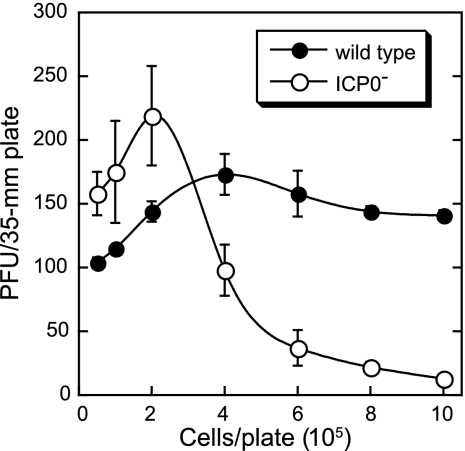
The plating efficiency of ICP0− viruses decreases as the cell density increases (5). Because a high cell density results in decreased plating efficiency, it is possible that reduced cell density resulting from the death of cells during Gln deprivation could result in enhanced plating efficiency.
To test this possibility, Vero cells were seeded in 35-mm plates with 5 × 103 to 1 × 106 cells/plate in complete DMEM. Twenty-four hours later, the monolayers were infected with 100 PFU/35-mm plate of wild-type or ICP0− virus. For cell densities of 2.5 × 104 and lower, there were not enough cells for plaques to form (Fig. 4). The number of plaques resulting from wild-type infection increased as the cell density increased to 4 × 105 cells/plate and then decreased very slightly. The plating efficiency of the ICP0− virus increased as the cell density increased to 2 × 105 cells/plate and then decreased sharply as cell density increased. For the Gln deprivation experiments presented in Fig. 1 to 3, 35-mm plates were seeded with 1.5 × 105 cells/plate. These results demonstrate that cell death due to Gln deprivation cannot account for the increase in plating efficiency.
FIG. 4.
The enhanced plating efficiency of an ICP0− virus following Gln deprivation is not due to reduced cell density. Vero cells were seeded at initial densities of 5 × 103 to 1 × 106 cells/35-mm plate. Twenty-four hours later, the monolayers were infected with 100 PFU/plate of wild-type (KOS) or ICP0− (n212) virus in complete DMEM. After a 1-h infection, monolayers were overlaid with complete DMEM containing methyl cellulose. Plaques were counted after a 5-day incubation at 37°C. Plaques did not form in monolayers seeded with 2.5 × 104 or fewer cells. Data points represent the average numbers of PFU/plate in three independent experiments, and error bars represent the standard deviations.
We have shown previously that heat shock and UV irradiation are stresses that result in enhanced plating efficiency of an ICP0− virus (5). Both of these stresses are able to induce reactivation of wild-type HSV-1 from latency in humans (25). Together, these observations show a possible correlation between treatments that complement an ICP0− virus and those that cause reactivation from latency. In this study, we have demonstrated that Gln deprivation results in enhanced plating efficiency of an ICP0− virus. An interesting question that arises is whether Gln deprivation can also cause reactivation from neuronal latency.
In humans, Gln levels decrease as a result of exercise, trauma, burns, extended bed rest, viral stress, and other stresses (1-4, 14, 23, 24, 26, 33, 34). Many of these conditions also cause reactivation from latency (12, 18, 28). Is Gln a common denominator in these observations? We are currently performing experiments to examine whether Gln deprivation can cause reactivation from latency.
Acknowledgments
This work was supported by National Institutes of Health grants RO1CA20260 from the National Cancer Institute and PO1NS35138 from the National Institute of Neurological Disorders and Stroke. R.M.B. was supported in part by grant T32AI706126 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 3 September 2008.
REFERENCES
- 1.Andrews, F. J., and R. D. Griffiths. 2002. Glutamine: essential for immune nutrition in the critically ill. Br. J. Nutr. 87(Suppl. 1)S3-S8. [DOI] [PubMed] [Google Scholar]
- 2.Biolo, G., R. Y. Fleming, S. P. Maggi, T. T. Nguyen, D. N. Herndon, and R. R. Wolfe. 2000. Inhibition of muscle glutamine formation in hypercatabolic patients. Clin. Sci. (London) 99189-194. [PubMed] [Google Scholar]
- 3.Biolo, G., B. Ciocchi, M. Lebenstedt, R. Barazzoni, M. Zanetti, P. Platen, M. Heer, and G. Guarnieri. 2004. Short-term bed rest impairs amino acid-induced protein anabolism in humans. J. Physiol. 558381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biolo, G., F. Zorat, R. Antonione, and B. Ciocchi. 2005. Muscle glutamine depletion in the intensive care unit. Int. J. Biochem. Cell. Biol. 372169-2179. [DOI] [PubMed] [Google Scholar]
- 5.Bringhurst, R. M., and P. A. Schaffer. 2006. Cellular stress rather than stage of the cell cycle enhances the replication and plating efficiencies of herpes simplex virus type 1 ICP0− viruses. J. Virol. 804528-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, W., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 634579-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 654078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai, W., and P. A. Schaffer. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 662904-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 677501-7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett, R. D. 1984. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 33135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D., A. Orr, and M. Elliott. 1991. High level expression and purification of herpes simplex virus type 1 immediate early polypeptide Vmw110. Nucleic Acids Res. 196155-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidler, P. E., B. T. Mackool, D. A. Schoenfeld, M. Malloy, J. T. Schulz III, R. L. Sheridan, and C. M. Ryan. 2002. Incidence, outcome, and long-term consequences of herpes simplex virus type 1 reactivation presenting as a facial rash in intubated adult burn patients treated with acyclovir. J. Trauma 5386-89. [DOI] [PubMed] [Google Scholar]
- 13.Gelman, I. H., and S. Silverstein. 1985. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 825265-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greig, J. E., D. G. Rowbottom, and D. Keast. 1995. The effect of a common (viral) stress on plasma glutamine concentration. Med. J. Aust. 163385-388. [DOI] [PubMed] [Google Scholar]
- 15.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer. 2001. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 756143-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 753240-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, A., and P. Agrawal. 1988. Glutamine decomposition in DMEM: effect of pH and serum concentration. Biotechnol. Lett. 10695-698. [Google Scholar]
- 18.McGill, S. N., and R. C. Cartotto. 2000. Herpes simplex virus infection in a paediatric burn patient: case report and review. Burns 26194-199. [DOI] [PubMed] [Google Scholar]
- 19.O'Hare, P., and G. S. Hayward. 1985. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J. Virol. 56723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Hare, P., and G. S. Hayward. 1985. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J. Virol. 53751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozturk, S. S., and B. O. Palsson. 1990. Chemical decomposition of glutamine in cell culture media: effect of media type, pH, and serum concentration. Biotechnol. Prog. 6121-128. [DOI] [PubMed] [Google Scholar]
- 22.Pardee, A. B. 1989. G1 events and regulation of cell proliferation. Science 246603-608. [DOI] [PubMed] [Google Scholar]
- 23.Rohde, T., D. A. MacLean, A. Hartkopp, and B. K. Pedersen. 1996. The immune system and serum glutamine during a triathlon. Eur. J. Appl. Physiol. Occup. Physiol. 74428-434. [DOI] [PubMed] [Google Scholar]
- 24.Rohde, T., D. A. MacLean, and B. K. Pedersen. 1998. Effect of glutamine supplementation on changes in the immune system induced by repeated exercise. Med. Sci. Sports Exerc. 30856-862. [DOI] [PubMed] [Google Scholar]
- 25.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 26.Rowbottom, D. G., D. Keast, and A. R. Morton. 1996. The emerging role of glutamine as an indicator of exercise stress and overtraining. Sports Med. 2180-97. [DOI] [PubMed] [Google Scholar]
- 27.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sainz, B., J. M. Loutsch, M. E. Marquart, and J. M. Hill. 2001. Stress-associated immunomodulation and herpes simplex virus infections. Med. Hypotheses 56348-356. [DOI] [PubMed] [Google Scholar]
- 29.Thompson, R. L., and N. M. Sawtell. 2006. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J. Virol. 8010919-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobey, R. A., and K. D. Ley. 1970. Regulation of initiation of DNA synthesis in Chinese hamster cells. I. Production of stable, reversible G1-arrested populations in suspension culture. J. Cell Biol. 46151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tritsch, G. L., and G. E. Moore. 1962. Spontaneous decomposition of glutamine in cell culture media. Exp. Cell Res. 28360-364. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox, C. L., R. L. Smith, R. D. Everett, and D. Mysofski. 1997. The herpes simplex virus type 1 immediate-early protein ICP0 is necessary for the efficient establishment of latent infection. J. Virol. 716777-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Windle, E. M. 2006. Glutamine supplementation in critical illness: evidence, recommendations, and implications for clinical practice in burn care. J. Burn Care Res. 27764-772. [DOI] [PubMed] [Google Scholar]
- 34.Wischmeyer, P. E. 2008. Glutamine: role in critical illness and ongoing clinical trials. Curr. Opin. Gastroenterol. 24190-197. [DOI] [PubMed] [Google Scholar]
- 35.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 696249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]