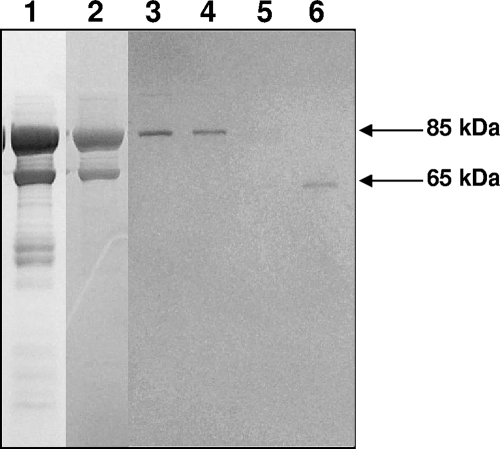
FIG. 2.
Purification of ClpB of M. pneumoniae. rClpB protein was purified through different columns as described in Materials and Methods, separated on SDS-PAGE gel, and Coomassie blue stained. Protein fractions were from a Ni-nitrilotriacetic acid column (lane 1), a Mono Q anion exchange column (lane 2), and a Superdex 200 gel filtration column eluted using a linear NaCl gradient (lanes 3 to 6).