FIG. 1.
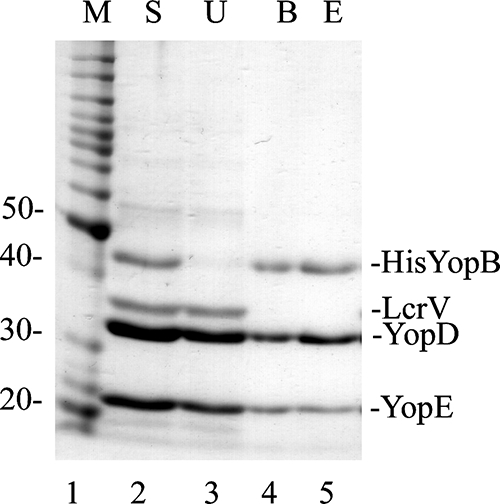
Purification of BDE translocation complex. A culture of Y. pseudotuberculosis YP46(pHis6-YopB) was grown under T3SS-inducing conditions in the presence of IPTG, and a culture supernatant containing secreted proteins was obtained following centrifugation. The culture supernatant was concentrated, supplemented with CHAPS (to 1 mM), and subjected to Ni2+ chelation affinity purification using His·Bind beads. Samples of the concentrated supernatant (S; lane 2), unbound proteins (U; lane 3), proteins bound to the beads (B; lane 4), and proteins eluted from the beads with imidazole (E; lane 5) were analyzed by SDS-PAGE and GelCode Blue staining. Lane 1 contains a protein molecular weight (MW) standard (M), and corresponding MWs are shown on the left. The positions of His-YopB, LcrV, YopD, and YopE are indicated on the right.
