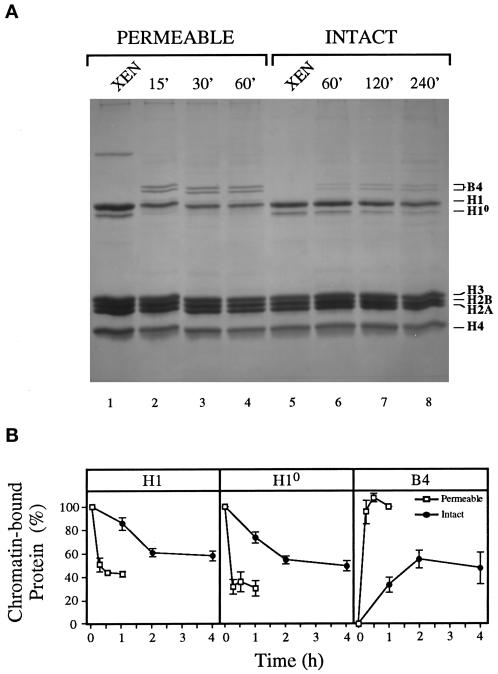
Figure 4.
An intact nuclear envelope reduces the remodeling of erythrocyte chromatin by egg extract. (A) Permeable and intact erythrocyte nuclei were incubated in extract for various times as indicated. Incubated and unincubated (XEN) nuclei were then diluted and sedimented, and the chromatin-associated proteins were extracted with acid, resolved by SDS-PAGE, and visualized with Coomassie blue. The positions of the embryonic linker histone B4, somatic linker histones H1 and H10, and core histones H3, H2B, H2A, and H4 are indicated. (B) The chromatin-bound H1, H10, and B4 proteins were quantitated by densitometry and normalized with the core histones in each sample shown in A. Shown are the mean percentages of each chromatin-bound protein, along with the SEM, from three separate experiments in which three different extracts were used. The amounts of H1 and H10 on unincubated XEN and the amount of B4 assembled on the chromatin of permeable nuclei after 60 min were designated as 100% bound protein.