Figure 1.
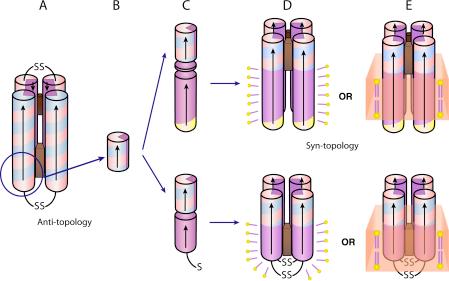
Schematic drawing of the AP family design and assembly. De novo designed maquettes are built from α-helices patterned along the helical axis with polar and non-polar residues: positive residues are colored blue, negative are red, polar uncharged are yellow and non-polar residues are purple. The incorporation of heme is displayed by a brown box. The HP domains of the AP maquettes described here are based on the structured diheme four-α-helical bundle HP1 that consists of two antiparallel dihelices (A). The HP1 dihelices are covalently linked by disulfide bond at the end of the flexible loops. AP1 (top) and AP3 (bottom) maquettes utilized only 14 residues close to the N-terminus and excluded the loops (blue circle). The abstracted HP domain (B) was linked to an LP domain either via flexible linker (AP1, top) or directly (AP3, bottom) (C). The polar/non-polar arrangement of the residues is designed (see also Figure 2) to drive assembly of four monomeric peptides to form a parallel four-α-helix bundle with two domains of sharply different polarity that confer amphiphilic character of the entire maquette. AP maquettes cannot be monodispersed and soluble in aqueous solution but they readily co-assemble with detergents to form micelles (D) or with lipids to form membranes (E).
