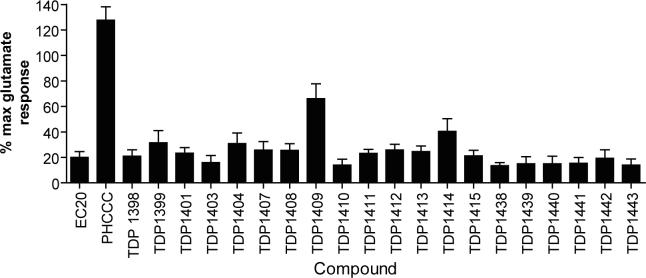
Figure 1. Chemical modifications of the PHCCC scaffold depicted here do not result in improved potency or efficacy for potentiation of mGluR4 activity.
Structures of synthesized compounds are shown in Supplemental Figure 1. Chemical synthesis is described in Supplemental Methods. Compounds 1a-e and 1k-p were synthesized according to the method of Annoura et al. (Annoura et al., 1998). Compounds 1f-j, 1l, and 1q were synthesized from 1g by the same methods. Compound 1g was prepared from 2-hydroxyacetophenone as described in Silva et al (Silva et al., 1998). A 30 μM final concentration of each compound was added to human mGluR4/Gqi5 cells; 2 ½ minutes later a submaximal (EC20) concentration of glutamate was added and changes in calcium-mediated fluorescence were measured. Results represent mean ± S.E.M. of three independent experiments performed in triplicate.