Table 2.
Potencies of Selected Arylstibonic Acids and Camptothecina
| compound | structure |
C0.5 (μM) |
|
|---|---|---|---|
| hTopob | vTopo | ||
| camptothecin |
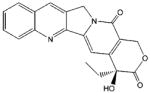
|
1.2 ± 0.5
(n = 1) |
~100c |
| P6954 |
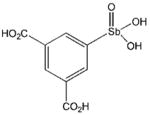
|
6.6 ± 0.4
(n = 3.6 ± 0.7) |
1.4 ± 0.6
(n = 1) |
| P6964 |
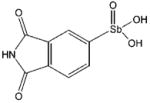
|
6.5 ± 0.7
(n = 3.9 ± 1.5) |
≥ 6 |
| P6982 |
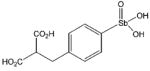
|
3.4 ± 0.3
(n = 2.0 ± 0.4) |
≥ 5 |
| 13759 |
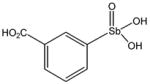
|
~ 6 d | 0.21 ± 0.07
(n = 1) |
Reactions were carried out in the presence of 14 nM pUC19 plasmid DNA substrate and 1 nM hTopo or vTopo.
Values in parentheses are Hill coefficients.
ref #
This compound shows activation and inhibition. The value is the apparent concentration for 50% inhibition.
