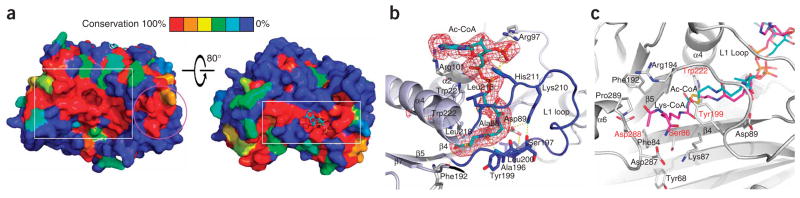
Figure 2.
Detailed view of the Rtt109–acetyl-CoA structure. (a) Surface conservation of the Rtt109 HAT domain among the first four sequences in Supplementary Figure 1. The color-coding indicates the degree of conservation as shown in the bar key. Left: the box highlights a patch of high surface conservation possibly involved in histone substrate binding; the circle highlights another conserved patch of surface conservation on the α8-α9 segment. Right: the box highlights the highly conserved acetyl-CoA binding site. (b) The acetyl-CoA binding site. Acetyl-CoA is shown in stick model with CPK coloring and with a simulated annealing omit map (omitting acetyl-CoA) contoured at 2.5σ. Hydrogen bonds are shown as yellow dashed lines. (c) The putative histone H3K56 substrate binding site. Residues proximal to the active site are shown as stick models in CPK coloring in a cartoon of the active site, with potential catalytic residues labeled in red. The Lys-CoA inhibitor from the p300–Lys-CoA structure (magenta) is overlaid for reference to indicate the putative H3K56 entry site.