Table 2.
Pd-Catalyzed Borylation of Aryl Halides with Pinacol Borane.a
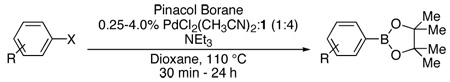 | |||||
|---|---|---|---|---|---|
| Entry | Aryl Halide | Pd (mol%) | Time | Yieldb | |
| 1 | X = I | 1.0 | 30 min | 94 | |
| 2 | X = I | 0.10 | 5 h | 91 | |
| 3 | X = Br | 1.0 | 1 h | 97 | |
| 4 | X = Cl | 3.0 | 24 h | 96c | |
| 5 | 1.0 | 3 h | 85 | ||
| 6 | X = Br | 1.0 | 4 h | 84 | |
| 7 | X = Cl | 3.0 | 24 h | 62c | |
| 8 | 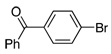 |
1.0 | 5 h | 70 | |
| 9 | 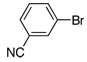 |
1.0 | 3 h | 57 | |
| 10 | 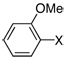 |
X = Br | 2.0 | 4 h | 89 |
| 11 | X = Cl | 4.0 | 24 h | 51c | |
| 12 | 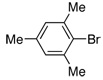 |
2.0 | 4 h | 90 | |
| 13 | 2.0 | 4 h | 68 | ||
| 14 | 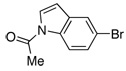 |
2.0 | 4 h | 97 | |
| 15 | 2.0 | 4 h | 74 | ||
| 16 | 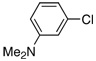 |
3.0 | 24 h | 80c | |
| 17 | 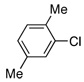 |
3.0 | 24 h | 87c | |
| 18 | 3.0 | 24 h | 59c | ||
| 19 | 3.0 | 24 h | 73c | ||
Reaction Conditions: 1 equiv of aryl or heteroaryl halide, 1.5 equiv of pinacol borane, 3 equiv of NEt3, 1,4-Dioxane (0.60 mL/mmol halide), cat. PdCl2(CH3CN)2, 1:Pd = 4:1.
Isolated yield based upon an average of two runs.
NEt3 (1.00 mL/mmol halide) used as solvent instead of 1,4-Dioxane.
