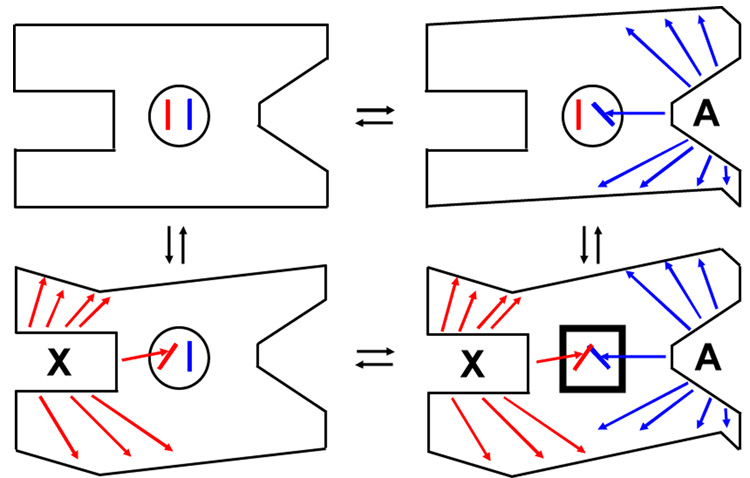
Figure 2.
A schematic of the four protein complexes of Figure 1. These simplified illustrations demonstrate how some ligand-elicited changes in the protein structure will be relevant to allostery, but others will not. They also show why allostery is only realized in the ternary complex. Ligand dependent structural changes are indicated by arrows and change in the exterior boarder of the protein. Structural changes associated with A binding are blue and those associated with X binding are red. The region with a crucial allosteric role is in the middle of the protein. The heavy square in XEA highlights allosteric changes resulting from the representative steric clash of levers.