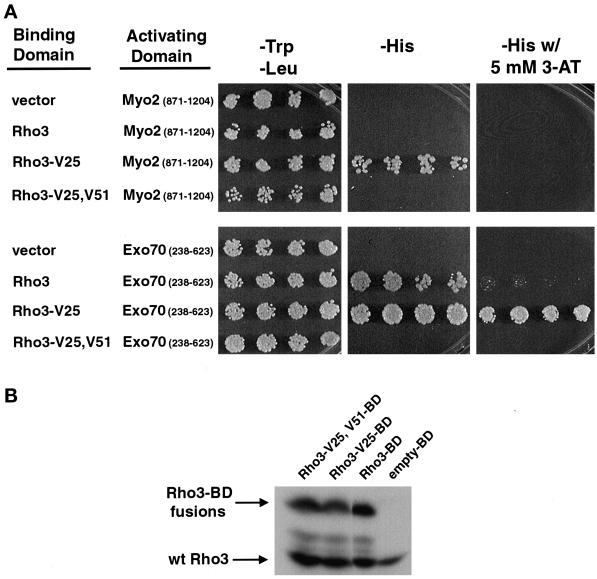
Figure 7.
Two-hybrid analysis of the effect of the V51 mutation on the interaction of Rho3 with Myo2 and Exo70. (A) Two-hybrid analysis of Rho3, Rho3-V25, and Rho3-V25,V51 mutants on the interaction with Myo2 and Exo70. Constructs containing GAL4BD-RHO3 and the GAL4AD-MYO2 (encoding residues 871-1024) or EXO70-GAL4AD (encoding residues 238–623) were transformed into PJ694α. Four independent transformants were replicated onto media selecting for both plasmids (−Trp, −Leu) or media that require activation of the HIS3 reporter (−His), and interactions were assayed by the ability of the two GAL4 fusions to activate the reporter gene HIS3. Growth on media lacking histidine or on media lacking histidine with 5 mM 3-aminotriazole (3-AT), which functions as an inhibitor of the histidine biosynthetic pathway and requires a higher level of HIS3 activation to support growth, is shown. (B) Western blot analysis of Rho3 protein levels in the two-hybrid transformants using affinity-purified α-Rho3 antibodies. Cotransformants were examined for the expression of the Gal4-binding domain (BD) fusion proteins. The endogenous Rho3 protein migrates at ∼29 kDa, and a larger fusion protein of the expected size is visible migrating at ∼48 kDa.