Abstract
Myelin, the insulating sheath made by extensive plasma membrane wrappings is dependent on the presence of highly adhesive molecules that keep the two sides of the membrane in tight contact. The Po glycoprotein (Po) is the major component of the peripheral nervous system (PNS) myelin of mammals. The exact role that Po protein has played in the evolution of myelin is still unclear, but several phylogenetic observations point to it as a crucial component in the development of myelin as a multi-lamellar membrane structure. Sharks, which appeared in evolution about 400 million years ago, are the first fully myelinated organisms. In this study we set out to investigate the expression pattern of shark myelin Po as a way of understanding how it might have played a role in the evolution of myelin in the central nervous system. We found that shark have more than two isoforms (32, 28 and 25kD), and that some of these might not be fully functional because they lack the domains known for Po homophilic adhesion.
Introduction
Myelin, in its compact form, is the insulating sheath that covers axons in the central and peripheral nervous system, allowing rapid nerve conduction. It consists of glial plasma membrane tightly wrapped around axons and devoid of any cytoplasmic fluid. Myelin compaction is dependent on the presence of highly adhesive molecules that keep the two sides of the membrane in tight contact. The Po glycoprotein (Po) is the major component of the peripheral nervous system (PNS) myelin of mammals. This protein has been shown to bind in a homophilic manner to an opposing membrane and is the molecule responsible for myelin compaction [D’Urso et al., 1990; Filbin et al., 1990; Giese et al., 1992]. The exact role that Po protein has played in the evolution of myelin is still unclear, but several phylogenetic observations point to it as a crucial component in the development of myelin as a multi-lamellar membrane structure. For instance, the Agnatha group, which lacks compact myelin, already shows Po immunoreactivity [Kirschner et al., 1989; Waehneldt, 1990]. However, although no Po has been reported among the sequenced genomes of sea squirt (Ciona), lancelet (Amphioxus) or any other invertebrate [Gould et al., 2005], recent analysis from Amphioxus libraries suggest that at least Po is present in the first chordates (Dr. Sauka-Spengler, Caltech, personal communication) [Sauka-Spengler et al., 2007]. Also, in elasmobranchs and teleost fish, Po is the major myelin protein component not only in the PNS but also in the central nervous system (CNS) [Waehneldt et al., 1986; Saavedra et al., 1989; Stratmann and Jeserich, 1995]. These observations have suggested to researchers that the transition between non-compact myelin to compact myelin parallels the appearance of Po in evolution.
Cloning of the shark Po revealed that this glycoprotein is conserved (~ 46%) throughout vertebrate evolution (fig. 1) and is the product of a single mRNA transcript [Waehneldt et al., 1987; Saavedra et al., 1989; Stratmann and Jeserich, 1995]. Furthermore, the cloning of a Po-like glycoprotein from trout CNS shows that it has about 50% sequence homology with shark and rat Po and also results from one mRNA transcript [Stratmann and Jeserich, 1995]. Indeed, Po in elasmobranchs also carries the same HNK-1 carbohydrate epitope as Po in mammals [Zand et al., 1991].
Figure 1. Myelin Po Sequence alignment and analysis.
Sequences from Genbank were aligned via the program of ClustalW. Sequences chosen for peptide antibody preparation are underlined. PoEx sequence shares some similarity with rodent Po. PoCy1 sequence has no homology with other Po proteins and PoCy2 corresponds to the cytoplasmic domain of Po which is highly conserved among most of the species we looked. The consensus key for the amino acids sequences is: * (single, fully conserved residue), :(semicolon, conservation of strong groups),.·(black dot, conservation of weak groups) and “blank” (no consensus).
In mammals only one Po isoform has been detected [Uyemura and Kitamura, 1991], while in sharks [Tai and Smith, 1983; Nunn et al., 1987; Saavedra et al., 1989] and chickens [Nunn et al., 1987] at least two isoforms are present. Bony fish also seem to have two Po-like proteins (IP1 and IP2) [Stratmann and Jeserich, 1995]. But the true identity of these Po-like proteins in shark has yet to be defined by methods more sophisticated than merely the determinations of molecular weight and type of glycosylation.
The value of studying these well known myelin proteins in sharks derives from the position that cartilaginous fish hold in the evolution of myelin. Sharks, which appeared in evolution about 400 million years ago, are the first fully myelinated organisms [Bakay and Lee, 1966; Waehneldt, 1990]. Before them, other organisms, like lampreys and earthworms, have glial membrane loosely wrapped around axons, though not true compact myelin [Bullock et al., 1984; Waehneldt et al., 1987]. Cartilaginous fish are considered to be the first ones to have compact myelin [Kitagawa et al., 1993]. All this implies that in the evolution of myelin several factors converged: presence of glial cells, presence of adhesive molecules and evolutionary advantage over non-compact myelin. Therefore, studying of the spatio-temporal expression of the major myelin protein in shark will help in our understanding of the evolution of myelin. In this study we set out to investigate the expression pattern of shark myelin Po as a way of understanding its contribution to the evolution of myelin in the central nervous system.
Materials and Methods
Po Antibodies
Rabbit antibodies against Po were raised by injection of purified bovine Po with Freunds adjuvant (RabA antibody) or of Po peptides with Titer-Max (peptide antibodies). The polyclonal antibody PoCy2, which recognizes the cytoplasmic domain of mouse Po has been described elsewhere [Gould et al., 1995]; it detects the full length Po protein. For the anti-Po peptide antibodies, two sequences from shark Po [Saavedra et al., 1989] and one from rat Po protein [Lemke et al., 1988] were selected to raise peptide antibodies (fig. 1). In order to raise antibodies that will be specific for shark only, we chose sequences were the similarity between shark and mouse was the lowest. Thus western blots using PoEx or PoCy1 on rat myelin did not show any cross-reactivity, confirming that these new antibodies are specific for shark Po.
Shark Nervous System Preparation
Shark nervous system regions from adult spiny dogfish (Squalus acanthias; Linnaeus, 1758) from Woods Hole Marine Biological Labs (MBL) were rapidly dissected and frozen on dry ice. Following the procedure reviewed and approved by the Animal Welfare Committee at MBL, the animals were anesthetized in 1:10,000 parts of MS222 in sea water until they were insensitive to touch and were then were killed by decapitation. The following regions were dissected: telencephalon (olfactory bulbs and cerebral hemispheres), diencephalon (epiphysis), mesencephalon (optic tectum), metencephalon (cerebellum), myelencephalon (medulla), and trigeminal nerves [Wischnitzer, 1993; Butler and Hodos, 2005]. Shark embryos collected from pregnant Squalus acanthias were staged based on fetal length as described in Ballard for shark embryos that are fully developed, though not yet fully grown [Ballard, 1993]. We collected and immediately froze brains from 4.0cm, 4.4cm, 9cm, and 22cm embryos. Crude membrane fractions were prepared by homogenizing each region in 4 volumes of 1X-SDS Laemli sample buffer (2% SDS, 10% Glycerol, 62.5mM Tris pH.6.8) in the presence of protease inhibitors (1μg/ml leupeptin, 2μg/ml antipain, 10μg/ml benzamidine, 1μg/ml pepstatin, 1μg/ml aprotinin, and 100μM PMSF). The protein content in each homogenate was estimated with a protein assay kit (Bio-Rad). Aliquots of the homogenates (depending on the figure the amounts ranged between 2–10μg of total protein in a 50μl volume) were loaded onto 12% polyacrylamide minigels (which have a 4% stacking gel to enhance protein resolution) in the presence of 1μl of β-mercaptoethanol and following electrophoresis (stacking gel run at 70mAmps and resolving gel at 35mAmp), transferred to polyvinylidene fluoride (PVDF) membranes (at 0.6Amps for 18hrs at 4°C in the following transfer buffer conditions: 50mM Na2HPO4 (anhydrous), 2mM EDTA, 0.025%SDS that was pH to 5.5 with conc. HCl. Blots were immunostained with 1:3000 dilutions of each different antibody. Shark Po was detected by secondary anti-rabbit antibody coupled either to alkaline phosphatase or horseradish peroxidase (1:20,000), and visualized by BCIP/NBT or ECL following manufacturer instructions (KPL) respectively.
Myelin Preparation
Myelin was isolated from the brains of spiny dogfish (Squalus acanthias, Linnaeus, 1758) by conventional procedure [Norton and Poduslo, 1973]. The spinal cord was homogenized in 0.25M sucrose with protease inhibitors (1μg/ml leupeptin, 2μg/ml antipain, 10μg/ml benzamidine, 1μg/ml pepstatin, 1μg/ml aprotinin, and 100μM PMSF) and the concentration brought up to 1.4M sucrose by adding 2.8M sucrose. The sample was overlaid with 0.85M sucrose and then 0.25M sucrose. The crude myelin was collected from the 0.25M/0.85M interface after spinning at 40,000g for 12 hr, osmotically shocked with water and spun again at 40,000g for 1hr. The myelin pellet was solubilized in 1X-SDS Laemli’s buffer (2% SDS, 10% Glycerol, 62.5mM Tris pH.6.8).
Endo F Treatment
80μg of shark (Squalus acanthias) spinal cord myelin fraction were boiled in 400μl of EndoF lysis buffer (50mM Tris pH8.6, 25mM EDTA, 0.1%SDS and 0.5%NP-40) in the presence of 2μl of β-mercaptoethanol. A cocktail of protease inhibitors (1μg/ml leupeptin, 2μg/ml antipain, 10μg/ml benzamidine, 1μg/ml pepstatin, 1μg/ml aprotinin, and 100μM PMSF) was added once the mixture cooled to room temperature, and 1μl (1U/ml) of EndoF (Boehringer-Manheim) was added. The mixture was incubated overnight at 37°C. Then 8 volumes of acetone were added to precipitate protein, and the mixture was cooled for 2 hours at −20°C. The proteins were pelleted by centrifugation at 5000rpm for 5 min in a Sorvall bench-top unit. Acetone was removed, and once the precipitate was dry, the proteins were re-suspended in 30μl of SDS sample buffer and 5μg of total protein was run on the gel.
Immunohistochemistry
Chiloscyllium punctatum (Müller and Henle, 1838) shark cases were harvested from the Long Beach Aquarium of the Pacific and the embryos collected at different developmental stages [Ballard, 1993], the youngest collected embryos were 3cm when they have developed most of their nervous system and they look like very small sharks; the oldest embryos were 10cm, a stage at which they show a lot of physical activity and look like their adults counterparts. Embryos were fixed in Carnoy’s (70% ethanol, 20% formaldehyde and 10% glacial acetic acid) overnight at 4°C and kept in 70% ethanol at −20°C until histology preparation. In order to get good morphology, embryos needed very extensive dehydration steps (about 1 day per alcohol grade) and two full days in histosol for clearing. The tissues were then immersed in hot paraffin (McCormick Scientific Paraplast Plus) in a vacuum oven for two days before preparing the blocks and sectioning 10–12μm thickness. Sections were re-hydrated in histosol and a graded series of ethanol washes (histosol, 100, 90, 70, 50 and 25% ethanol washes in water) and then equilibrated in PBS (Dulbecco’s) before blocking in PBS containing 10% expired FBS and 1% Triton X-100 for 1hr. Primary antibodies (rabbit polyclonal anti-myelin Po and anti-myelin basic protein [Gould, 1992]) were added in a 1:500 dilution in PBS and slides were incubated overnight at 4°C. After washing the sections in PBS for at least 20 minutes, secondary antibodies (Alexa fluoroprobes conjugated to anti-rabbit or anti-mouse IgG, Invitrogen) were added for 30 min and washed in PBS for immuno-fluorescence visualization and cover-slipped with Permount. Pictures of sections were taken using Axiovision LE software (Zeiss™) with an AxioCam black and white camera attached to a Zeiss AxioimagerA1 upright fluorescent microscope and assembled into figures 7 and 8 using Adopbe Photoshop 7.
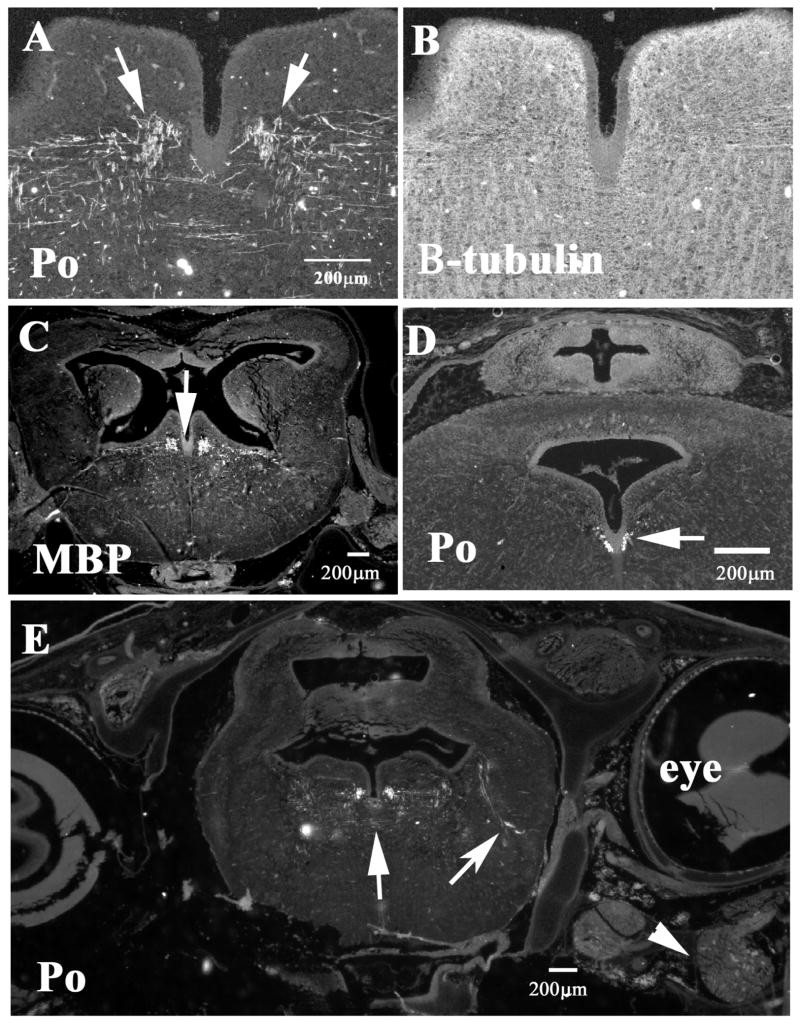
Figure 7. Myelin protein expression in CNS of shark embryos.
Immuno-histochemistry of 6cm shark embryos showed that although myelination is not widespread in the CNS, there are distinguishable myelinated tract in the shark embryo brain. A and B show tectal region double stained for Po (A) and beta-tubulin III (neuron specific, B), arrows point to myelinated fibers. Hindbrain regions close to the cerebellum (C–D) show fewer Po-positive fibers, D, than MBP in a consecutive section (7C). Arrows point to the fibers positive for MBP and Po. Midbrain section at the level of the eye shows more Po-positive fibers as well myelinated tracts reaching laterally (arrow). Arrowhead points to the trigeminal ganglia.
Figure 8. Myelin protein expression in spinal cord and ganglia embryos.
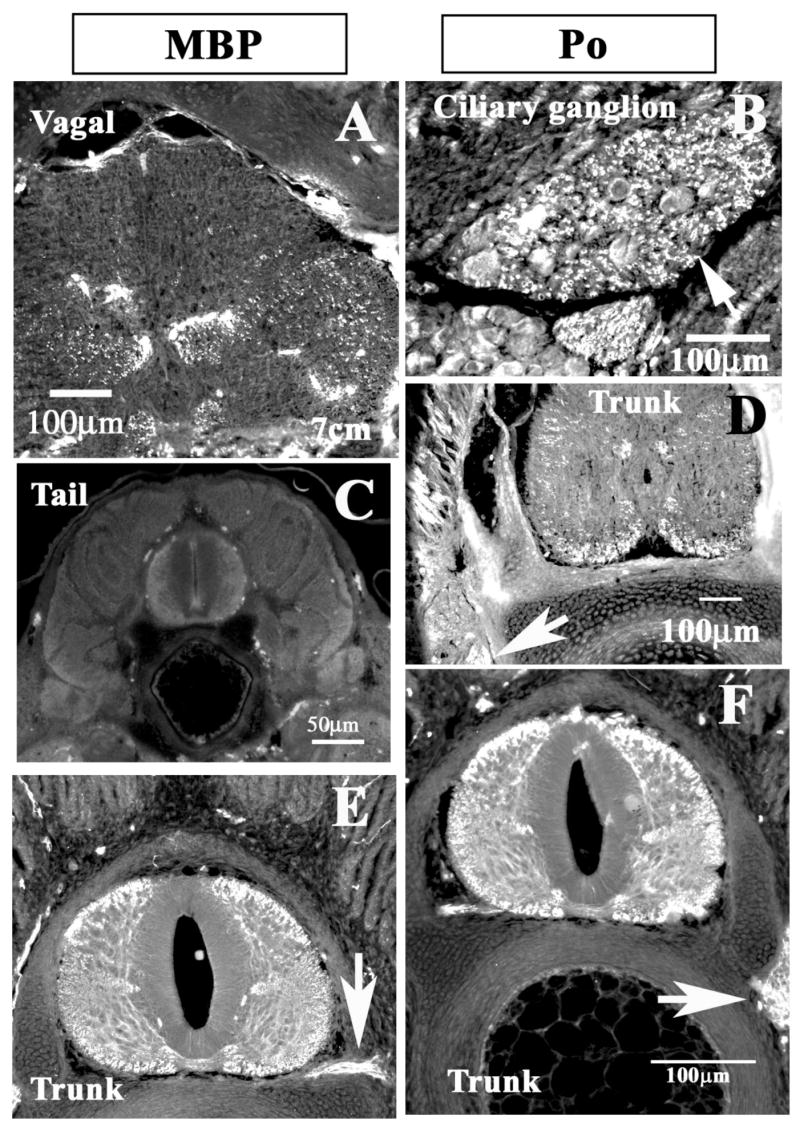
Shark embryo sections (3cm A, C, D) and 7cm (B, E, F) were immunostained for MBP (A,C and E) or Po (B, D and F) proteins. A corresponds to spinal cord at the vagal/medulla level, notice extensive MBP positive tracts (spinal cord ventral area in 8A), but at the tail level there was none (8C for MBP). Cranial ganglia myelination is more extensive and sooner (arrows in B). At the trunk levels (D), we observed Po-positive fibers in the ventral spinal cord but none in sensory ganglia (arrow). In older embryos (E and F) MBP and Po expression respectively was widespread and extensive, including spinal nerve (arrow in E) and ganglia (arrow in F).
Results
Shark Po Antibodies Recognize Different Isoforms of Po
When the antibody that recognizes the full-length Po (RabA in fig. 2) was used in a Western blot of purified shark myelin, two major protein bands were observed. These bands match the molecular weights of the two Po isoforms (28 and 32kD) previously reported for shark [Saavedra et al., 1989]. A smaller band of lower-molecular-weight (25kD) protein, not strongly stained, was also apparent. This band has not been previously reported for shark Po.
Figure 2. Shark Myelin Po isoforms.
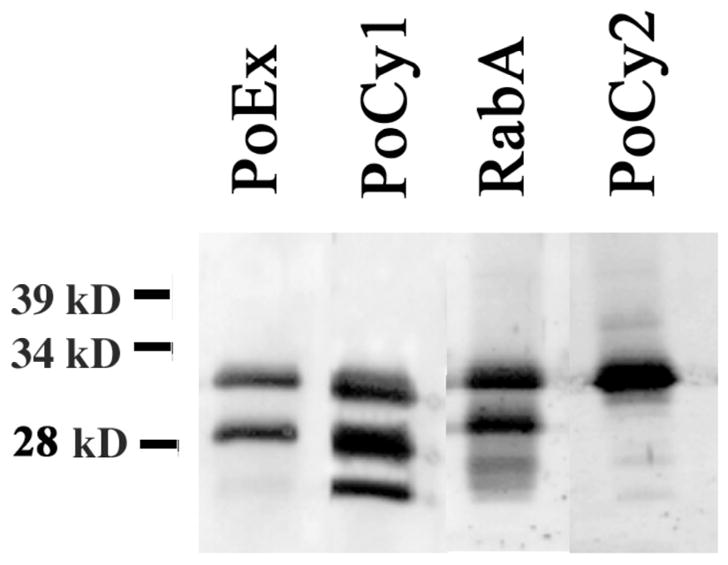
Western blot of purified shark myelin (5μg) showed several isoforms for Po. An antibody raised specifically to the shark cytoplasmic region (PoCy1) shows three bands for Po, while antibodies to the extracellular domain (PoEx) or a polyclonal antibody raised against purified Po (RabA) showed two strong bands and a fainter one. The antibody raised against the highly conserved cytoplasmic end of Po showed only one band. A total of 4ug of protein from purified myelin were loaded on each lane.
Immuno-detection using PoCy1 in a sample from the same purified shark myelin revealed three prominent bands at apparent molecular weights of 25, 28 and 32kD (fig. 2). This peptide antibody, specific for the cytoplasmic domain of shark Po, recognized the same two proteins previously observed with an antibody that recognizes full-length Po as well as a third isoform that matches the smaller band seen in that same Western. A Western blot with the PoEx antibody, raised against the N-terminus sequence of shark Po, showed only the two major 28 and 32kD protein bands. The lack of detection of the smaller 25kD band with this antibody suggests that this Po isoform is missing at least part of the corresponding N-terminus peptide sequence from its extracellular domain.
The Western blot with PoCy2 antibody detected only the major band at 32kD (fig. 2). This peptide antibody corresponds to the C-terminus of rat Po, and is 65% homologous to the shark Po C-terminus. The fact that PoCy2 did not detect the 25 and 28kD protein suggests that these two protein bands are different isoforms of shark Po that lack this end terminus region.
Our finding of several isoforms in the CNS and PNS from shark with different truncations has several explanations: that there has been some proteolytic degradation during sample preparation; or that they are due to endogenous proteolytic intermediates present in the lysosomes. The possibility that protein degradation took place during the myelin preparation and that it can account for the presence of several protein isoforms with decreasing molecular weights can be disregarded because all the procedures were performed with freshly dissected tissues and these were kept at 4°C and in the presence of suitable amounts of protease inhibitors. Second, we did not see any changes in the molecular weight of the major band or an enrichment of the lower band when the lysates were purposively left overnight at room temperature before running them on a gel (data not shown). Third, these gel patterns have been consistent and reproducible under many experimental conditions, no matter method of sample preparation (to corroborate the uniqueness of these isoforms, we also ran the proteins under non-denaturing conditions, without β–mercaptoethanol) or the antibody used in the Western. The second possibility was that the Po isoforms found in shark are proteolytic intermediates present in the lysosomes. First, if that were the case, we should have observed a more varied number of smaller isoforms in our Westerns, which we did not. Second, we should have also observed varying and/or different amounts of proteins for all the isoforms; but we found nearly equal and reproducible amounts of the full length (32kD) as well of the intermediate isoforms (28 and 25kD) throughout our Westerns.
Glycosylation of Shark Po Isoforms
The myelin Po protein is known to be heavily glycosylated [Uyemura et al., 1981], our finding of three different shark Po protein bands suggests that these might be due to different levels of glycosylation. To determine if this may be the case, we performed an Endoglycase F treatment on the myelin fraction to remove all the sugar residues from shark’s myelin. A Western blot of this fraction showed that these 3 isoforms are fully glycosylated (fig. 3), since each band decreased equally by approximately 6% of their apparent molecular weight after de-glycosylation, instead of becoming one single band of 25kD. This finding suggests that the three different isoforms we observed may be to be products of different Po variants rather than differences in glycosylation.
Figure 3. Endo F treatment of myelin Po.
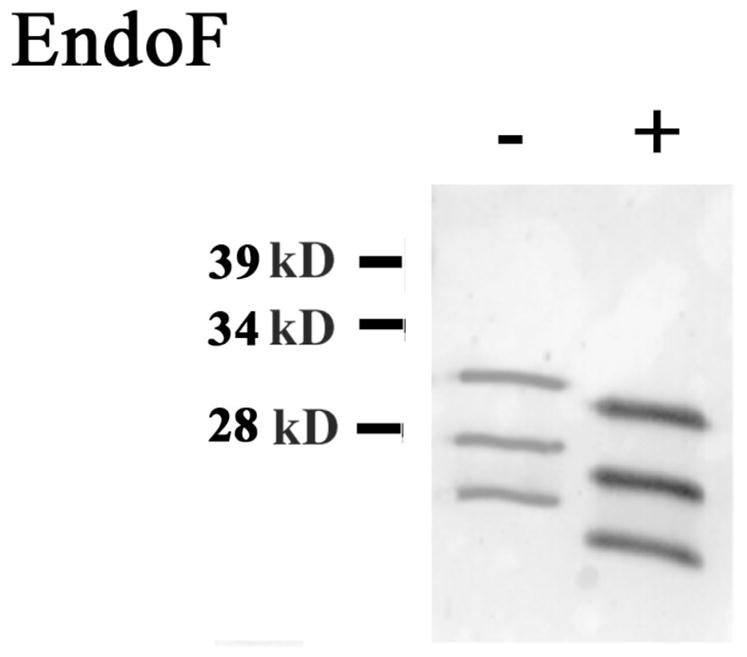
Purified shark myelin was treated with an enzyme (EndoF) to remove all carbohydrates. Western blot showed that enzyme treated Po(+) isoforms run about 6% lower than fully glycosylated Po (−). A total of 4μg of protein from purified myelin were loaded on each lane.
Regional Distribution of Po Isoforms
The regional distribution of these Po isoforms in adult shark nervous system was studied similarly by doing Western blots from solubilized telencephalon, diencephalon, optic tectum, cerebellum and medulla. We found that these CNS regions share the three major isoforms (25, 28 and 32kD) also recognized by PoCy1 in total purified myelin (fig. 4). Using PoEx antibody showed only the two major bands in these samples, as with total purified shark myelin observed in figure 2 (data not shown). The telencephalon showed the lowest levels of Po, with faint amounts of the 25kD isoform, while cerebellum and diencephalon showed the highest levels of all three isoforms (~50% higher than telencephalon).
Figure 4. Po isoform distribution in shark brain.
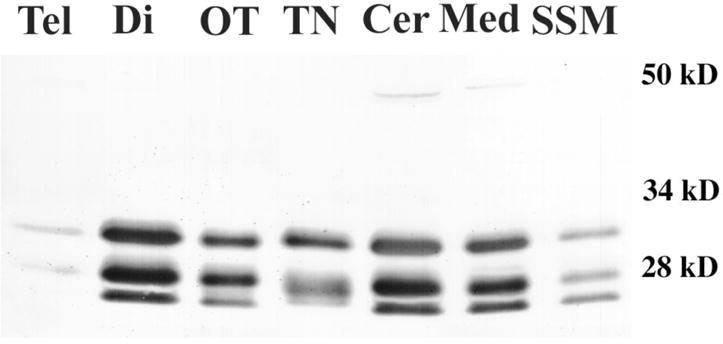
Western blots with PoCy1 from different brain regions showed that there was an unequal distribution of shark Po in different areas. 8μg of total brain lysates were prepared from dissected areas and ran on a SDS-PAGE gel. Tel (telencephalon), Di (diencephalon), OT (optic tectum), TN (trigeminal nerve), Cer (cerebellum), Med (medulla) and SSM (spinal cord myelin).
We also looked at Po protein in shark PNS using trigeminal nerve. We found that apparently the expression of Po in the PNS is different from that in CNS. As shown in figure 5, trigeminal nerve has a marked 32kD band and a second band running at ~27kD. In order to determine if it was due to different levels of glycosylation, we repeated the process of removing all the carbohydrates from the sample with EndoF. We observed that as expected for a fully glycosylated Po, the band ran 6% lower after EndoF treatment though still at a clearly different weight compared with our standard myelin from spinal cord (fig. 5).
Figure 5. Glycosylation of CNS and PNS shark myelin.
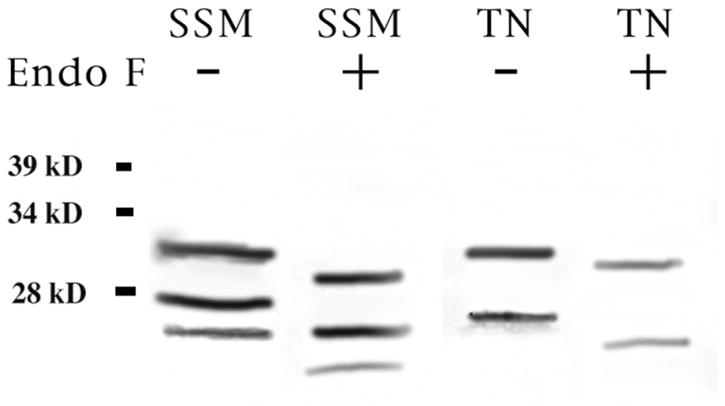
Purified shark myelin (5μg) was treated with an enzyme to remove all carbohydrates (Endo F). Western blot showed that enzyme treated Po (+) isoforms run about 6% lower than fully glycosylated Po (−) for both CNS (SSM, shark spinal cord myelin) and PNS (TN, trigeminal nerve myelin). TN myelin showed only two of the three isoforms found in CNS.
Developmental Expression of Shark’s Po Isoforms
Finally, in order to find out if these distinct Po isoforms are regulated or equally expressed throughout development, whole brain from sharks at different stages of development were analyzed. Figure 6 shows Western blots of Squalus acanthias shark at embryonic stages of 4, 4.4, 9 and 22cm. Levels of Po glycoprotein were detectable only in the 22cm embryos. We were able to observe only the 28 and 32kD isoforms, indicating that the 25kD isoform is not present during the initial stages of shark myelin formation. After over-exposure using ECL detection methods (Enhanced Chemiluminescence) we were able to observe that the 9cm embryo had low levels of the 28 and 32kD. A similar finding of fewer isoforms during development has been reported for trout larvae [Jeserich et al., 1990; Stratmann and Jeserich, 1995]. In addition, we observed a 34kD isoform in the 22cm embryo (arrow in fig. 6), not present in adult shark brain after extensive over-exposures using Enhanced Chemiluminescence’s detection (data not shown).
Figure 6. Developmental expression of myelin Po.
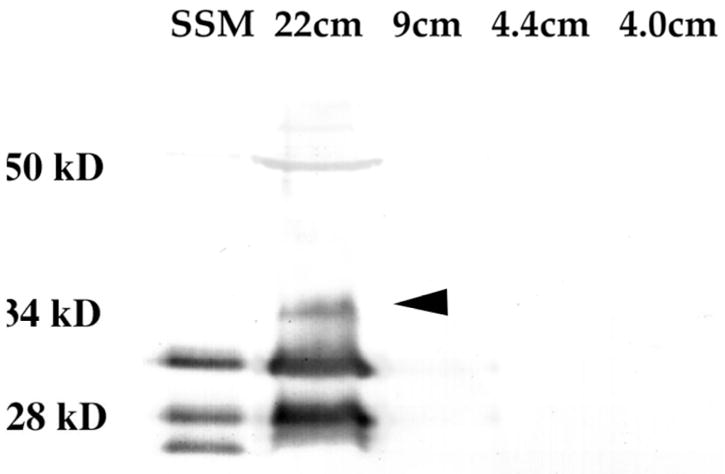
Shark brains from embryos at different developmental stages (4, 4.4 9 and 22cm embryos) were solubilized and ran on an SDS-PAGE gel. Western blot was done with the PoCy1 antibody that recognizes all three shark isoforms. Only the oldest embryo showed significant amounts of Po, and had three isoforms, the 32 and 28kD as well as a novel 34kD (arrowhead). The total protein loaded was: 16μg of protein for the 4, 4.4 and 9cm embryos, 4μg for the 22cm embryo and 2μg for the positive control SSM (shark spinal cord myelin).
We further corroborated that Po is not found in the very early stages of shark development (5cm) by performing immunohistochemistry of shark embryo sections (embryos at 5cm of a different species: Chiloscyllium punctatum) using a RatPo antibody that is capable of detecting Po in fixed tissue (fig. 7A, 7D and 7E; data for younger embryos, smaller than 5cm, not shown). The earliest time that we were able to immunostain for Po was in embryos at 5cm stage) and onwards [Ballard, 1993]. In the youngest embryos we observed very few myelinated tracts, but in older embryos (10cm) there were more Po positive fibers running laterally in the midbrain (fig. 7A, 7D and 7E). Meanwhile in the midbrain area there was robust and widespread neurofilament expression, this is a marker for mature neurons (fig. 7B). Because the staining for Po was not striking in these younger embryos, we performed immunostaining for myelin basic protein (MBP) another marker for myelination (fig. 7C). We noticed that MBP expression was detectable at earlier stages and in more fibers compared with Po in the Chiloscyllium embryos (compare fig. 8C with 8D). We were able to detect smaller amounts of Po and larger amounts of MBP in stage 32 spinal cords and hindbrain (fig. 8D and A), suggesting that MBP expression may come before Po. Later on in development (7cm embryos) we observed robust and comparable amounts of Po and MBP expression in the spinal cords of the embryos as well as in the PNS: ciliary ganglion, spinal nerves and sensory ganglions (fig. 8B, D, E, F).
Discussion
Research on non-mammalian myelin Po expression suggested the existence of two to four Po-like glycoproteins in shark and fish CNS [Tai and Smith, 1983; Tai and Smith, 1984; Saavedra et al., 1989]. Our study demonstrates for the first time that those shark glycoproteins, sometimes referred to as IP1-IP4 [Jeserich and Waehneldt, 1986], are indeed Po isoforms and that there is an additional isoform that was not considered Po in the past. We were able to establish this fact by the use of specific antibodies against shark’s Po peptide sequences.
Previous studies showed that there were two Po isoforms in another elasmobranch species, the gummy shark (Mustelus antarcticus), but they did not identify the smallest, 25kD glycoprotein in whole brain extracts (likely IP4) as Po [Tai and Smith, 1984]. This discrepancy may be explained by: a) the fact that we observed that this isoform is present in myelin at much lower amounts (~50%) than the other two, b) that Tai and Smith did not use antibodies for the detection of these Po proteins in their blots and c) that they studied a carcharhiniform shark, which has a brain type different from the shark species we used, a squalomorph, that has a simpler laminar brain type. Although we used different shark species for immunohistochemical detection of Po and MBP during development, there is published and unpublished data with Mustelus canis, which indicates that in terms of expression of these proteins, the results are the same as the ones presented here [Gould, 1992; Gould et al., 1995]. Furthermore, Squalus (which we used for the Western blot analysis) is considered a primitive shark and Chiloscyllium (which we used for immunohistochemistry) is considered a more modern one. However, looking at their brains, the gross morphology is very different, e.g. the cerebellum of Squalus is smooth and Mustelus (a species more like Chiloscyllium, has a very convoluted brain). In spite of these overall differences, the cellular structures are quite similar and when we used Mustelus myelin, we got similar labeling.
The Po isoforms we detected in shark (25, 27, 28 and 32kD) are fully glycosylated, as shown by their shift (~6%) in mobility in SDS gels, and are differentially expressed throughout the shark’s nervous system. We found that samples from several CNS regions (telencephalon, diencephalon, optic tectum, cerebellum, medulla and spinal cord) all share the same three Po isoforms, whereas the PNS (trigeminal nerve) contains only the 27 and 32kD. This two PNS bands are not the product of different degrees of glycosylation but rather suggests that at least in trigeminal nerve there may be a separate, distinct isoform of slightly lower molecular weight (27kD) than the second one (28kD) widely present in the CNS.
To date, only one Po mRNA transcript from shark was isolated from a cDNA library [Saavedra et al., 1989]. Our finding of several isoforms in the CNS and PNS from shark with different truncations has several explanations: that either these isoforms are due to differential splicing of Po mRNA transcripts or that these isoforms are due to posttranslational modification of the full-length shark Po. Up until now the best characterized of the myelin Po proteins, besides mammalians, is the one in fish. In the trout researchers found only one mRNA [Stratmann and Jeserich, 1995]. At least two isoforms have been reported for another shark, though these reported isoforms do not have the same molecular weight as the ones reported in this paper [Tai and Smith, 1983; Tai and Smith, 1984; Zand et al., 1991]. In other words, elasmobranchs seem to have a series of proteolytically degraded forms of myelin Po that fish or mammals do not present.
All these results lead to the suggestion that Po undergoes proteolytical modification. This event is not uncommon and happens to be an effective mechanism of physiological regulation [Neurath, 1989]. It has been observed to occur at the cytoplasmic as well as extracellular N-terminus of several adhesion molecules and receptors [Ozawa and Kemler, 1990; Covault et al., 1991; O’Bryan et al., 1995]. E-Cadherin adhesiveness requires a precise endogenous cleavage of a peptide at its N-terminus domain [Ozawa and Kemler, 1990]. Slit2 is a well known chemorepellant molecule that is also proteolytically cleaved in the cell [Nguyen Ba-Charvet et al., 2001]. Moreover, there is evidence in sharks of post-translational modification of MBP [Deibler et al., 1975; Zand et al., 2001]. Thus, it is quite feasible that during myelination, different proteases may be activated that will modify the expression of Po as it happens for MBP. However, there is yet no strong evidence towards a splice hypothesis or a proteolytic one.
The PNS and CNS myelin organization of cartilaginous and higher vertebrates depends on the same myelinating glial cells: oligodendrocytes for CNS and Schwann cells for the PNS [Bakay and Lee, 1966; Long et al., 1968; Schweigreiter et al., 2006]. However, the differentiated oligodendrocytes of mammals do not express Po at all but rather the PLP/DM20 glycoproteins [Milner et al., 1985]. Cartilaginous and bony fish have PLP/DM20-like proteins [Kitagawa et al., 1993], but these proteins do not seem to play the role in myelin compaction that is observed in terrestrial vertebrates like mammals [Boison et al., 1995; Yoshida and Colman, 1996]. Due to the high levels of Po in both PNS and CNS of non-mammalian vertebrates, it has been hypothesized that Po is responsible for myelin compaction in these organisms. However, at some point during evolution, between cartilaginous fish and reptiles, Po stopped being expressed in CNS, and PLP/DM20 came to play a major role in the myelin formation of higher vertebrates.
Our observations of several isoforms of Po with different truncations could shed some light on the change of role of Po, as well the “phenotypically silent drop-out of Po from the terrestrial vertebrate CNS” [Yoshida and Colman, 1996]. Throughout evolution protein modifications of Po in CNS myelin, like the proteolytically cleaved products we present in this paper could have made it less capable of sustaining compact myelin, allowing the adhesive PLP/DM20-like glycoproteins, already present as potential adhesive proteins, to take over that role in myelogenesis. Wong and Filbin have already shown that a truncated rat Po at its cytoplasmic terminus is incapable of supporting homophilic adhesion of transfected CHO cells and that, furthermore, it has a dominant negative effect on the adhesion of the full-length Po [Wong and Filbin, 1996]. The isoform of Po with such an effect is missing the same sequence recognized by the PoCy2 antibody. This sequence is absent as well in the 28, 27 and 25kD isoforms in shark’s Po. Maybe the post-translational modifications of full-length Po in elasmobranchs stripped it of its adhesive properties, leaving an open door for the PLP/DM20 protein to substitute for Po as the major adhesive molecule in the CNS. More than a “silent drop” of Po as the major adhesive component in CNS myelin, it was a “takeover” by the PLP/DM-20 molecules. It remains to be seen whether each of the identified isoforms of shark Po can support homophilic adhesion. The fact that this protein is so highly conserved throughout evolution suggests that those forms lacking the cytoplasmic domain may not support homophilic adhesion, or at least not in the same fashion as the full-length protein.
The pattern for Po shown in this paper is very different from the one found in higher vertebrates, where Po is present only in the PNS, and only as a single isoform with slight variations in its levels of glycosylation. The current result of several Po isoforms, spatially and developmentally regulated is a novel finding for this major myelin glycoprotein.
Acknowledgments
We give very special thanks to Marie T. Filbin and her lab members for their support when carrying out some of these experiments and for providing the specific shark antibodies used in these paper. We thank Cindy Malone and Sonsoles de Lacalle for useful discussions and Peter Rudy for technical assistance. This work was partly supported by an NIH-MBRS SCORE-5S06GM048680-13.
References
- Bakay L, Lee JC. Ultrastructural changes in the edematous central nervous system. 3. Edema in shark brain. Arch Neurol. 1966;14:644–660. doi: 10.1001/archneur.1966.00470120076011. [DOI] [PubMed] [Google Scholar]
- Ballard WW, Jean Mellinger, Henri Lechenault. A Series of Normal Stages for Development of Scyliorhinus canicula, the Lesser Spotted Dogfish (Chondrichthyes: Scyliorhinidae) J Exp Zool. 1993;267:318–336. [Google Scholar]
- Boison D, Bussow H, D’Urso D, Muller HW, Stoffel W. Adhesive properties of proteolipid protein are responsible for the compaction of CNS myelin sheaths. J Neurosci. 1995;15:5502–5513. doi: 10.1523/JNEUROSCI.15-08-05502.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative vertebrate neuroanatomy : evolution and adaptation. 2. Hoboken, N.J.: Wiley-Interscience; 2005. [Google Scholar]
- Covault J, Liu QY, el-Deeb S. Calcium-activated proteolysis of intracellular domains in the cell adhesion molecules NCAM and N-cadherin. Brain Res Mol Brain Res. 1991;11:11–16. doi: 10.1016/0169-328x(91)90015-p. [DOI] [PubMed] [Google Scholar]
- D’Urso D, Brophy PJ, Staugaitis SM, Gillespie CS, Frey AB, Stempak JG, Colman DR. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron. 1990;4:449–460. doi: 10.1016/0896-6273(90)90057-m. [DOI] [PubMed] [Google Scholar]
- Deibler GE, Martenson RE, Kramer AJ, Kies MW. The contribution of phosphorylation and loss of COOH-terminal arginine to the microheterogeneity of myelin basic protein. J Biol Chem. 1975;250:7931–7938. [PubMed] [Google Scholar]
- Filbin MT, Walsh FS, Trapp BD, Pizzey JA, Tennekoon GI. Role of myelin P0 protein as a homophilic adhesion molecule. Nature. 1990;344:871–872. doi: 10.1038/344871a0. [DOI] [PubMed] [Google Scholar]
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–576. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Gould RM, Fannon AM, Moorman SJ. Neural cells from dogfish embryos express the same subtype-specific antigens as mammalian neural cells in vivo and in vitro. Glia. 1995;15:401–418. doi: 10.1002/glia.440150405. [DOI] [PubMed] [Google Scholar]
- Gould RM, Morrison HG, Gilland E, Campbell RK. Myelin tetraspan family proteins but no non-tetraspan family proteins are present in the ascidian (Ciona intestinalis) genome. Biol Bull. 2005;209:49–66. doi: 10.2307/3593141. [DOI] [PubMed] [Google Scholar]
- Gould RM, Spivack WD, Gilland E, Pant HC, Tseng D. Localization of Myelin Proteins in the Developing Shark Spinal Cord. Biol Bull. 1992;183:358–359. doi: 10.1086/BBLv183n2p358. [DOI] [PubMed] [Google Scholar]
- Jeserich G, Muller A, Jacque C. Developmental expression of myelin proteins by oligodendrocytes in the CNS of trout. Brain Res Dev Brain Res. 1990;51:27–34. doi: 10.1016/0165-3806(90)90255-w. [DOI] [PubMed] [Google Scholar]
- Jeserich G, Waehneldt TV. Characterization of antibodies against major fish CNS myelin proteins: immunoblot analysis and immunohistochemical localization of 36K and IP2 proteins in trout nerve tissue. J Neurosci Res. 1986;15:147–158. doi: 10.1002/jnr.490150204. [DOI] [PubMed] [Google Scholar]
- Kirschner DA, Inouye H, Ganser AL, Mann V. Myelin membrane structure and composition correlated: a phylogenetic study. J Neurochem. 1989;53:1599–1609. doi: 10.1111/j.1471-4159.1989.tb08558.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Sinoway MP, Yang C, Gould RM, Colman DR. A proteolipid protein gene family: expression in sharks and rays and possible evolution from an ancestral gene encoding a pore-forming polypeptide. Neuron. 1993;11:433–448. doi: 10.1016/0896-6273(93)90148-k. [DOI] [PubMed] [Google Scholar]
- Lemke G, Lamar E, Patterson J. Isolation and analysis of the gene encoding peripheral myelin protein zero. Neuron. 1988;1:73–83. doi: 10.1016/0896-6273(88)90211-5. [DOI] [PubMed] [Google Scholar]
- Long DM, Bodenheimer TS, Hartmann JF, Klatzo I. Ultrastructural features of the shark brain. Am J Anat. 1968;122:209–236. doi: 10.1002/aja.1001220204. [DOI] [PubMed] [Google Scholar]
- Milner RJ, Lai C, Nave KA, Lenoir D, Ogata J, Sutcliffe JG. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985;42:931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Neurath H. Proteolytic processing and physiological regulation. Trends Biochem Sci. 1989;14:268–271. doi: 10.1016/0968-0004(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21:4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Nunn DJ, LeBlanc AC, Mezei C. A 42 K protein of chick sciatic nerve is immunologically related to PO protein of peripheral nerve myelin. Neurochem Res. 1987;12:377–384. doi: 10.1007/BF00993248. [DOI] [PubMed] [Google Scholar]
- O’Bryan JP, Fridell YW, Koski R, Varnum B, Liu ET. The transforming receptor tyrosine kinase, Axl, is post-translationally regulated by proteolytic cleavage. J Biol Chem. 1995;270:551–557. doi: 10.1074/jbc.270.2.551. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol. 1990;111:1645–1650. doi: 10.1083/jcb.111.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra RA, Fors L, Aebersold RH, Arden B, Horvath S, Sanders J, Hood L. The myelin proteins of the shark brain are similar to the myelin proteins of the mammalian peripheral nervous system. J Mol Evol. 1989;29:149–156. doi: 10.1007/BF02100113. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, Bronner-Fraser M. Ancient evolutionary origin of the neural crest gene regulatory network. Dev Cell. 2007;13:405–420. doi: 10.1016/j.devcel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Schweigreiter R, Roots BI, Bandtlow CE, Gould RM. Understanding myelination through studying its evolution. Int Rev Neurobiol. 2006;73:219–273. doi: 10.1016/S0074-7742(06)73007-0. [DOI] [PubMed] [Google Scholar]
- Stratmann A, Jeserich G. Molecular cloning and tissue expression of a cDNA encoding IP1--a P0-like glycoprotein of trout CNS myelin. J Neurochem. 1995;64:2427–2436. doi: 10.1046/j.1471-4159.1995.64062427.x. [DOI] [PubMed] [Google Scholar]
- Tai FL, Smith R. Shark CNS myelin contains four polypeptides related to the PNS protein Po of higher classes. Brain Res. 1983;278:350–353. doi: 10.1016/0006-8993(83)90270-6. [DOI] [PubMed] [Google Scholar]
- Tai FL, Smith R. Comparison of the major proteins of shark myelin with the proteins of higher vertebrates. J Neurochem. 1984;42:426–433. doi: 10.1111/j.1471-4159.1984.tb02695.x. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Horie K, Suzuki M, Kitamura K. Glycosylation of myelin glycoproteins in peripheral nerve via lipid intermediates. Neurochem Res. 1981;6:959–968. doi: 10.1007/BF00965027. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Kitamura K. Comparative studies on myelin proteins in mammalian peripheral nerve. Comp Biochem Physiol C. 1991;98:63–72. [PubMed] [Google Scholar]
- Waehneldt TV. Phylogeny of myelin proteins. Ann N Y Acad Sci. 1990;605:15–28. doi: 10.1111/j.1749-6632.1990.tb42377.x. [DOI] [PubMed] [Google Scholar]
- Waehneldt TV, Matthieu JM, Stoklas S. Immunological evidence for the presence of myelin-related integral proteins in the CNS of hagfish and lamprey. Neurochem Res. 1987;12:869–873. doi: 10.1007/BF00966308. [DOI] [PubMed] [Google Scholar]
- Waehneldt TV, Stoklas S, Jeserich G, Matthieu JM. Central nervous system myelin of teleosts: comparative electrophoretic analysis of its proteins by staining and immunoblotting. Comp Biochem Physiol B. 1986;84:273–278. doi: 10.1016/0305-0491(86)90076-3. [DOI] [PubMed] [Google Scholar]
- Wischnitzer S. Atlas and dissection guide for comparative anatomy. 5. New York: W.H. Freeman and Co.; 1993. p. 76. [Google Scholar]
- Wong MH, Filbin MT. Dominant-negative effect on adhesion by myelin Po protein truncated in its cytoplasmic domain. J Cell Biol. 1996;134:1531–1541. doi: 10.1083/jcb.134.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Colman DR. Parallel evolution and coexpression of the proteolipid proteins and protein zero in vertebrate myelin. Neuron. 1996;16:1115–1126. doi: 10.1016/s0896-6273(00)80138-5. [DOI] [PubMed] [Google Scholar]
- Zand D, Hammer J, Gould R, Quarles R. High expression of the HNK-1/L2 carbohydrate epitope in the major glycoproteins of shark myelin. J Neurochem. 1991;57:1076–1079. doi: 10.1111/j.1471-4159.1991.tb08260.x. [DOI] [PubMed] [Google Scholar]
- Zand R, Jin X, Kim J, Wall DB, Gould R, Lubman DM. Studies of posttranslational modifications in spiny dogfish myelin basic protein. Neurochem Res. 2001;26:539–547. doi: 10.1023/a:1010921230859. [DOI] [PubMed] [Google Scholar]