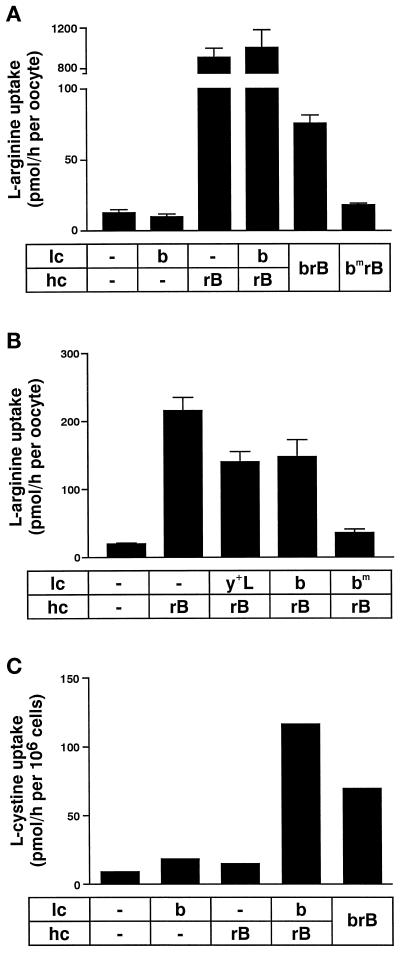
Figure 3.
Both hrBAT and mb0,+AT are required for the expression of b0,+-type transport function. (A) No l-arginine uptake over background level was observed when oocytes expressed the b0,+AT light chain (b) alone. hrBAT (rB) alone, which associated with endogenous oocyte light chains, induced a high transport rate. Coexpression of the b0,+AT light chain with rBAT did not modify the transport rate significantly. The fusion protein hrBAT-mb0,+AT (brB) induced in oocytes an l-arginine uptake that was severalfold higher than that observed with fusion protein containing the mutant b0,+ATE244Q light chain moiety (bmrB) or that of water-injectedoocytes. (B) Oocytes were first injected with the light chain cRNA for mb0,+AT, mb0,+ATE244Q, or my+LAT. After 2 d of expression, hrBAT cRNA was injected and l-arginine uptake was measured 24 h later. The preexpression of the mutant light chain mb0,+ATE244Q nearly fully inhibited l-arginine uptake expression induced by rBAT, presumably by efficiently competing with functional endogenous chains for association with rBAT. Means of 12 oocytes ± SEM are shown. (C) Mouse M1 cell lines expressing rBAT, b0,+AT, both, or the fusion protein hrBAT-mb0,+AT (brB) were seeded on plastic dishes, and the uptake of l-cystine (2.5 μM) was measured. A single experiment with one cell line each is shown. Several cell lines were tested for single and double transfectants and gave comparable results. Uptakes significantly higher than by untransfected control cells were obtained only in double transfectant and fusion protein–expressing cell lines (Bauch and Verrey, unpublished data).