Abstract
Background
While guidelines recommend measuring fasting lipids for initial screening of adults without cardiovascular disease (CVD), recent studies suggest that nonfasting triglycerides may be superior to fasting. Whether fasting status alters associations of non-triglyceride lipids with CVD is unclear.
Methods and Results
In a prospective study of 26,330 healthy women (19,983 fasting; 6,347 nonfasting), associations of baseline lipids with incident CVD (754 fasting; 207 nonfasting) were examined over 11-year follow-up. Except for triglycerides, lipid concentrations differed minimally (<5%) fasting versus nonfasting. However, stronger associations with CVD were noted for fasting total cholesterol (adjusted fasting HR 1.22 per 1-standard deviation increment, 95% CI, 1.14−1.30; nonfasting HR 1.07, 0.93−1.21), LDL cholesterol (fasting HR 1.21, 1.13−1.29; nonfasting HR 1.00, 0.87−1.15), apolipoprotein B100 (fasting HR 1.36, 1.27−1.45; nonfasting HR 1.20, 1.05−1.36), non-HDL cholesterol (fasting HR 1.29, 1.21−1.38; nonfasting HR 1.15, 1.01−1.31), and apolipoprotein B100/A-1 ratio (fasting HR 1.39, 1.30−1.48; nonfasting HR 1.18, 1.09−1.27). Compared with fasting levels, nonfasting HDL cholesterol, apolipoprotein A-1, and total/HDL cholesterol ratio had similar associations, and triglycerides had stronger association, with CVD. Significant interactions were seen for LDL cholesterol and apolipoprotein B100/A-1 ratio with fasting status (P for interaction=0.03 and <0.001, respectively).
Conclusions
This study demonstrates that HDL cholesterol, triglycerides, total/HDL cholesterol ratio, and apolipoprotein A-1 predict CVD when measured nonfasting. By contrast, total, LDL, and non-HDL cholesterol, in addition to apolipoprotein B100 and B100/A-1 ratio, provide less useful CVD risk information when nonfasting, despite small changes in their concentrations. Guidelines for lipid screening may need to consider these differences.
Keywords: Lipids, apolipoproteins, women
Current guidelines recommend measurement of a fasting lipid profile for cardiovascular risk assessment.1,2 Lipids are traditionally measured after an 8- to 12-hour fast to minimize the influence of postprandial lipemia.3 Ingestion of a typical fat-containing meal results in higher triglyceride levels and smaller changes in low-density-lipoprotein (LDL) and high-density-lipoprotein (HDL) cholesterol.4 The third report of the NCEP Adult Treatment Panel (ATP III) recommends that initial screening should include a fasting lipid profile that includes total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides.1 The guidelines allow for the measurement of total and HDL cholesterol in the nonfasting state,1 since levels of these two lipids are altered minimally when measured in fasting or nonfasting blood. 5,6 Non-HDL cholesterol, a secondary target of therapy in ATP III, may also be used in the nonfasting state.1
In apparent contrast to these guidelines, three studies have suggested that nonfasting triglycerides may better or similarly predict cardiovascular disease (CVD) events than fasting levels.7-9 It has been increasingly recognized that postprandial responses, such as those relating to glucose and triglyceride metabolism, may trigger a number of proatherosclerotic and prothrombotic processes, including inflammation, oxidative stress, and vasoconstriction.10 It is unknown if nonfasting status alters the association of non-triglyceride lipids and apolipoproteins with CVD. However, if postprandial effects do not substantially weaken the association of nontriglyceride lipids and apolipoproteins with CVD, then measurement of nonfasting lipids may have many practical advantages for clinical practice. Therefore, we conducted this study in a large prospective cohort of initially healthy women in order to 1) evaluate changes in lipids and apolipoproteins as a function of time after a typical meal, and 2) determine whether fasting compared with nonfasting status alters the association of these lipids and apolipoproteins with incident CVD.
METHODS
Study Population
Study participants were enrolled in the Women's Health Study, a recently completed randomized, double-blinded, placebo-controlled clinical trial of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer in US female healthcare professionals.11-13 Eligible participants were apparently healthy women, ages 45 years or older, who were free of self-reported cardiovascular disease or cancer at study entry (1992−1995), with follow-up for incident CVD through February 2006. At the time of enrollment, participants gave written informed consent, completed questionnaires on demographics, medical history, medications, and lifestyle factors. They were also asked to provide a blood sample. Participants were requested, but not required, to have the sample drawn in the morning before eating, and reported the number of hours since their last meal before the blood draw and the time of day for the blood draw. In total, 27,748 women had baseline measurements on the lipids and apolipoproteins of interest. After excluding 1,418 women due to missing data on the time since last meal, there were 26,330 women for analysis. The study was approved by the institutional review boards of the Brigham and Women's Hospital (Boston, Mass). The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Baseline Plasma Measurements
EDTA blood samples were obtained at the time of enrollment and stored in vapor phase liquid nitrogen (−170°C). In a laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program, baseline samples were thawed and analyzed for standard lipids and apolipoproteins. Total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol were assayed directly using reagents from Genzyme Corporation (Cambridge, Mass) and Roche Diagnostics (Indianapolis, Ind) using a Hitachi 911 autoanalyzer. Apolipoproteins A1 and B100 were measured using immunoturbidimetric assays (DiaSorin, Stillwater, Minn).
Definition of Fasting Status
Participants whose last meal was 8 hours or more prior to their blood draw comprised the fasting sample (N=19,983), and those who had eaten within 8 hours of their blood draw comprised the nonfasting sample (N=6,347). The study population was also divided into groups according to time since last meal by 2-hour intervals of <2 (N=991), 2 to <4 (N=2,782), 4 to <6 (N=1,702), 6 to <8 (N=872), 8 to <10 (N=1,321), 10 to <12 (N=3,490), 12 to <14 (N=8,550), 14 to <16 (5,196), and 16 or more hours (N=1,426).
Ascertainment of CVD Events
The primary endpoint of interest was a composite endpoint of incident CVD (nonfatal myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, nonfatal stroke, or cardiovascular death). During the 11-year follow-up period, women reported the endpoints of interest on follow-up questionnaires every 6 or 12 months. All events were adjudicated by an end points committee.
Statistical Analysis
Statistical analyses were performed using STATA version 8.2 (STATA Corporation, College Station, Texas). Statistical comparisons between the fasting and nonfasting groups were obtained from student's t-tests for continuous variables expressed as means, from Kruskal-Wallis tests for variables expressed as medians, and chi-square tests for categorical variables. The levels of the lipids and apolipoproteins were examined as a function of time since the last meal divided into 2-hour intervals.
Following guidelines from the Department of Health and Human Services,14 lipid biomarkers were divided into quintiles based on the distribution among women not taking hormone replacement. Because the distributions differed between fasting and nonfasting participants for some of the lipid measurements, quintile cut-points were defined separately in each of the fasting and nonfasting samples. To address whether the results may differ on the basis of the cut-points uses, analyses were also performed per 1-standard deviation (1-SD) increment in each lipid variable. The 1-SD increments were similar for fasting and nonfasting samples. In order to examine the predictive value of levels of lipids and apolipoproteins for CVD risk depending on the time since the last meal divided into 2-hour intervals, analyses were repeated per 1-SD increments within strata of time since last meal by 2-hour intervals. Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) according to these quintiles and per 1-SD increments.
To examine the extent to which each lipid or lipoprotein biomarker was associated with incident events, we considered each lipid variable in a separate model that adjusted for non-lipid risk factors (age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index). Analyses for triglycerides were additionally adjusted for total and HDL cholesterol based on prior work from this cohort.8 To address any potential confounding from time of day for the blood draw, we additionally adjusted the multivariable models for time of blood draw, which did not affect the findings. P value for linear trend was obtained using the median value for each quintile. All P-values were two-tailed. Finally, statistical tests for interaction between fasting status and each of the lipids or apolipoproteins in relation to incident CVD were obtained using likelihood ratio tests.
RESULTS
Table 1 shows the baseline characteristics of participants according to fasting or nonfasting status. Nonfasting women were slightly younger, had less prevalent hypertension, more diabetes, and were less likely to be postmenopausal than fasting women. Compared with fasting lipids, nonfasting lipids were modestly (by ∼1 to 5%) but statistically significantly lower for total cholesterol, LDL cholesterol, apolipoprotein B100, non-HDL cholesterol, total/HDL cholesterol ratio and apolipoprotein B100/A-1 ratio, with no significant difference for HDL cholesterol or apolipoprotien A-1. As anticipated, triglycerides were higher in the nonfasting women (by ∼15%).
Table 1.
Baseline Characteristics of Participants According to Fasting Status*
| Fasting N=19,983 | Nonfasting N=6,347 | P Value | |
|---|---|---|---|
| Age, mean (SD), y | 55.0 (7.2) | 53.8 (6.6) | <0.001 |
| Current smoking, % | 11.7 | 11.1 | 0.19 |
| Hypertension, % | 26.0 | 22.5 | <0.001 |
| Diabetes, % | 2.6 | 3.1 | 0.03 |
| Postmenopausal status, % | 55.6 | 50.8 | <0.001 |
| Postmenopausal hormone use, % | 43.6 | 44.2 | 0.34 |
| Body mass index, mean (SD), kg/m2 | 25.9 (5.0) | 25.9 (5.0) | 0.72 |
| Lipid Concentrations, median (25th to 75th percentile), mg/dL | |||
| Total cholesterol | 209 (185−236) | 206 (181−234) | <0.001 |
| Low-density lipoprotein cholesterol | 123 (102−146) | 117 (97−140) | <0.001 |
| High-density lipoprotein cholesterol | 52 (43−62) | 52 (43−62) | 0.25 |
| Triglycerides | 115 (81−169) | 133 (93−196) | <0.001 |
| Apolipoprotein Concentrations, median (25th to 75th percentile), mg/dL | |||
| Apolipoprotein B100 | 103 (85−122) | 98 (82−118) | <0.001 |
| Apolipoprotein A-1 | 149 (133−168) | 150 (132−169) | 0.68 |
| Combined Lipid Measures, median (25th to 75th percentile) | |||
| Non-high-density lipoprotein cholesterol, mg/dL | 155 (130−183) | 152 (127−181) | <0.001 |
| Total/high-density lipoprotein cholesterol ratio | 4.0 (3.2−4.9) | 3.9 (3.2−4.9) | 0.03 |
| Apolipoprotein B100/A-1 ratio | 0.68 (0.54−0.85) | 0.66 (0.52−0.82) | <0.001 |
Fasting defined as ≥8 hours time since last meal.
P values were obtained from student's t-tests for continuous variables expressed as means, from Kruskal-Wallis tests for variables expressed as medians, and chi-square tests for categorical variables.
During a median follow-up of 11.4 years, a total of 961 first CVD events occurred (3.34 events per 1000 person-years of follow-up), which affected 754 out of 19,983 fasting women (3.8%) and 207 out of 6,347 nonfasting women (3.3%). Associations of each lipid variable with incident CVD were examined according to nonfasting or fasting quintiles and per 1-SD increments, in separate Cox regression models which considered each lipid variable, one at a time, and adjusted for non-lipid risk factors (Table 2). Nonfasting levels of total cholesterol and LDL cholesterol were not associated with CVD (adjusted nonfasting HR per 1-SD increment in total cholesterol 1.07, 95% CI, 0.93−1.21, P=0.35; and for LDL cholesterol 1.00, 0.87−1.15, P=1.0). By contrast, fasting levels of both total cholesterol and LDL cholesterol were significantly associated with CVD (fasting HRs, respectively, of 1.22, 1.14−1.30, and 1.21, 1.13−1.29, P<0.001 for both).
Table 2.
Associations of Lipids and Apolipoproteins with Cardiovascular Disease According to Fasting Status
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for Trend | Per 1-SD | P Value | PInteraction | |
|---|---|---|---|---|---|---|---|---|---|
| Lipid Concentrations | |||||||||
| Total cholesterol | |||||||||
| Nonfasting N=6,347 | 1.00 | 0.88 (0.49−1.57) | 1.53 (0.92−2.55) | 1.10 (0.65−1.86) | 1.40 (0.85−2.30) | 0.12 | 1.07 (0.93−1.21) | 0.35 | 0.10 |
| Fasting N=19,983 | 1.00 | 1.50 (1.09−2.07) | 1.62 (1.18−2.22) | 2.08 (1.55−2.81) | 2.28 (1.70−3.06) | <0.001 | 1.22 (1.14−1.30) | <0.001 | |
| Low-density lipoprotein cholesterol | |||||||||
| Nonfasting | 1.00 | 0.96 (0.61−1.51) | 0.83 (0.52−1.32) | 0.93 (0.59−1.47) | 0.95 (0.61−1.48) | 0.88 | 1.00 (0.87−1.15) | 1.0 | 0.03 |
| Fasting | 1.00 | 1.16 (0.87−1.54) | 1.44 (1.10−1.89) | 1.76 (1.36−2.28) | 1.83 (1.42−2.37) | <0.001 | 1.21 (1.13−1.29) | <0.001 | |
| High-density lipoprotein cholesterol | |||||||||
| Nonfasting | 1.00 | 0.70 (0.47−1.04) | 0.46 (0.29−0.73) | 0.33 (0.20−0.53) | 0.42 (0.27−0.65) | <0.001 | 0.75 (0.64−0.89) | 0.001 | 0.65 |
| Fasting | 1.00 | 0.71 (0.57−0.89) | 0.74 (0.59−0.92) | 0.61 (0.49−0.78) | 0.49 (0.39−0.63) | <0.001 | 0.79 (0.72−0.86) | <0.001 | |
| Triglycerides | |||||||||
| Nonfasting | 1.00 | 1.08 (0.54−2.15) | 1.38 (0.73−2.62) | 1.96 (1.06−3.60) | 2.54 (1.41−4.59) | <0.001 | 1.22 (1.12−1.33) | <0.001 | 0.75 |
| Fasting | 1.00 | 1.55 (1.06−2.27) | 2.27 (1.59−3.22) | 2.08 (1.46−2.95) | 2.63 (1.87−3.70) | <0.001 | 1.23 (1.16−1.30) | <0.001 | |
| Triglycerides* | |||||||||
| Nonfasting | 1.00 | 1.01 (0.50−2.03) | 1.26 (0.65−2.43) | 1.69 (0.89−3.23) | 2.04 (1.03−4.04) | 0.007 | 1.17 (1.04−1.31) | 0.008 | 0.96 |
| Fasting | 1.00 | 1.34 (0.92−1.96) | 1.75 (1.22−2.51) | 1.42 (0.98−2.05) | 1.47 (1.00−2.16) | 0.66 | 1.07 (1.00−1.15) | 0.09 | |
| Apolipoprotein Concentrations | |||||||||
| Apolipoprotein B100 | |||||||||
| Nonfasting | 1.00 | 0.95 (0.53−1.71) | 1.00 (0.57−1.76) | 1.08 (0.63−1.86) | 1.77 (1.07−2.91) | 0.001 | 1.20 (1.05−1.36) | 0.007 | 0.12 |
| Fasting | 1.00 | 1.24 (0.88−1.74) | 1.54 (1.11−2.13) | 1.69 (1.23−2.32) | 2.61 (1.93−3.54) | <0.001 | 1.36 (1.27−1.45) | <0.001 | |
| Apolipoprotein A-1 | |||||||||
| Nonfasting | 1.00 | 1.26 (0.82−1.95) | 0.65 (0.39−1.08) | 0.49 (0.29−0.84) | 0.72 (0.47−1.12) | 0.03 | 0.86 (0.73−1.00) | 0.04 | 0.72 |
| Fasting | 1.00 | 0.87 (0.69−1.10) | 0.67 (0.52−0.86) | 0.66 (0.52−0.84) | 0.58 (0.46−0.73) | <0.001 | 0.82 (0.75−0.89) | <0.001 | |
| Combined Lipid Measures | |||||||||
| Non-high-density lipoprotein cholesterol | |||||||||
| Nonfasting | 1.00 | 0.65 (0.37−1.17) | 0.95 (0.57−1.59) | 1.03 (0.62−1.70) | 1.49 (0.93−2.37) | 0.004 | 1.15 (1.01−1.31) | 0.03 | 0.15 |
| Fasting | 1.00 | 1.49 (1.05−2.12) | 2.24 (1.62−3.10) | 2.22 (1.61−3.07) | 2.99 (2.18−4.10) | <0.001 | 1.29 (1.21−1.38) | <0.001 | |
| Total/high-density lipoprotein cholesterol ratio | |||||||||
| Nonfasting | 1.00 | 1.07 (0.63−1.82) | 0.90 (0.53−1.55) | 1.43 (0.87−2.35) | 2.36 (1.46−3.80) | <0.001 | 1.28 (1.15−1.44) | <0.001 | 0.38 |
| Fasting | 1.00 | 1.48 (1.10−2.00) | 1.73 (1.30−2.32) | 1.94 (1.45−2.59) | 2.90 (2.19−3.84) | <0.001 | 1.36 (1.27−1.45) | <0.001 | |
| Apolipoprotein B100/A-1 ratio | |||||||||
| Nonfasting | 1.00 | 0.76 (0.46−1.25) | 0.73 (0.44−1.22) | 1.05 (0.66−1.69) | 1.68 (1.08−2.61) | 0.001 | 1.18 (1.09−1.27) | <0.001 | 0.0006 |
| Fasting | 1.00 | 1.79 (1.32−2.41) | 1.96 (1.46−2.64) | 2.12 (1.59−2.84) | 3.29 (2.48−4.37) | <0.001 | 1.39 (1.30−1.48) | <0.001 |
Values shown are hazard ratios (95% confidence intervals) adjusted for age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index. P value for linear trend was obtained using the median value for each quintile. Pinteraction was obtained from likelihood ratio tests for interaction with fasting/nonfasting status and the lipid variable in relation in CVD.
Additionally adjusted for total and HDL cholesterol.
Stronger associations with CVD were also noted for fasting compared with nonfasting levels of apolipoprotein B100 (fasting HR 1.36, 1.27−1.45; nonfasting HR 1.20, 1.05−1.36), non-HDL cholesterol (fasting HR 1.29, 1.21−1.38; nonfasting HR 1.15, 1.01−1.31), and apolipoprotein B100/A-1 ratio (fasting HR 1.39, 1.30−1.48; nonfasting HR 1.18, 1.09−1.27). Fasting and nonfasting associations of HDL cholesterol, apolipoprotein A-1, and total/HDL cholesterol ratio with CVD were comparable. A statistically significant interaction was seen between fasting/nonfasting status and LDL cholesterol for incident CVD (P for interaction=0.03), and for apolipoprotein B100/A-1 ratio (P for interaction<0.001), with a borderline significant interaction between total cholesterol and CVD (P for interaction=0.10). Consistent with our prior report,8 both fasting and nonfasting triglycerides were positively associated with CVD, but further adjustment for total cholesterol and HDL cholesterol weakened the association of fasting triglycerides with CVD. Including time of day for blood draw in the multivariable models did not change our findings.
When we compared the association of fasting and nonfasting lipids and apolipoproteins in women grouped according to baseline use of postmenopausal hormones (Table 3), a similar pattern was observed. The associations of lipids and apolipoproteins with CVD were overall somewhat stronger in the women not taking hormones, but the effect of nonfasting status was similar to that seen in the entire cohort. A statistically significant interaction was seen between fasting/nonfasting status and apolipoprotein B100/A-1 for incident CVD in non hormone users (P for interaction<0.001), and a borderline significant interaction between LDL cholesterol and CVD in hormone users (P for interaction=0.06).
Table 3.
Associations of Lipids and Apolipoproteins with Cardiovascular Disease, According to Fasting Status and Postmenopausal Hormone Use
|
Non Hormone Users Nonfasting N=3,531 Fasting N=11,258 |
Hormone Users Nonfasting N=2,801 Fasting N=8,688 |
|||
|---|---|---|---|---|
|
Per 1-SD |
P Value |
Per 1-SD |
P Value |
|
| Lipid Concentrations | ||||
| Total cholesterol | ||||
| Nonfasting | 1.10 (0.93−1.30) | 0.28 | 1.03 (0.84−1.26) | 0.81 |
| Fasting | 1.27 (1.17−1.38) | <0.001 | 1.14 (1.02−1.27) | 0.02 |
| Low-density lipoprotein cholesterol | ||||
| Nonfasting | 1.06 (0.89−1.27) | 0.49 | 0.90 (0.72−1.13) | 0.37 |
| Fasting | 1.26 (1.16−1.37) | <0.001 | 1.14 (1.01−1.27)** | 0.03 |
| High-density lipoprotein cholesterol | ||||
| Nonfasting | 0.68 (0.54−0.86) | 0.002 | 0.83 (0.66−1.05) | 0.12 |
| Fasting | 0.74 (0.65−0.84) | <0.001 | 0.83 (0.73−0.95) | 0.005 |
| Triglycerides | ||||
| Nonfasting | 1.22 (1.10−1.37) | <0.001 | 1.24 (1.08−1.43) | 0.002 |
| Fasting | 1.27 (1.18−1.36) | <0.001 | 1.17 (1.07−1.29) | 0.001 |
| Triglycerides* | ||||
| Nonfasting | 1.12 (0.96−1.30) | 0.15 | 1.26 (1.06−1.49) | 0.01 |
| Fasting | 1.07 (0.97−1.18) | 0.19 | 1.06 (0.94−1.19) | 0.38 |
| Apolipoprotein Concentrations | ||||
| Apolipoprotein B100 | ||||
| Nonfasting | 1.25 (1.06−1.47) | 0.009 | 1.14 (0.92−1.41) | 0.24 |
| Fasting | 1.40 (1.28−1.53) | <0.001 | 1.31 (1.17−1.46) | <0.001 |
| Apolipoprotein A-1 | ||||
| Nonfasting | 0.80 (0.64−1.00) | 0.05 | 0.90 (0.73−1.11) | 0.34 |
| Fasting | 0.74 (0.65−0.84) | <0.001 | 0.89 (0.79−1.00) | 0.05 |
| Combined Lipid Measures | ||||
| Non-high-density lipoprotein cholesterol | ||||
| Nonfasting | 1.19 (1.01−1.41) | 0.04 | 1.09 (0.89−1.34) | 0.41 |
| Fasting | 1.35 (1.24−1.47) | <0.001 | 1.21 (1.09−1.35) | 0.001 |
| Total/high-density lipoprotein cholesterol ratio | ||||
| Nonfasting | 1.34 (1.16−1.55) | <0.001 | 1.21 (1.00−1.47) | 0.05 |
| Fasting | 1.42 (1.31−1.53) | <0.001 | 1.25 (1.11−1.40) | <0.001 |
| Apolipoprotein B100/A-1 ratio | ||||
| Nonfasting | 1.18 (1.08−1.29) | <0.001 | 1.19 (0.98−1.44) | 0.08 |
| Fasting | 1.42 (1.31−1.53)** | <0.001 | 1.32 (1.17−1.48) | <0.001 |
Values shown are hazard ratios (95% confidence intervals) adjusted for age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index. P value for linear trend was obtained using the median value for each quintile.
Additionally adjusted for total and HDL cholesterol.
Likelihood ratio test for interaction with fasting/nonfasting status: LDL cholesterol for hormone users, P for interaction=0.06; apolipoprotein B100/A-1 for non hormone users, P for interaction=0.0002.
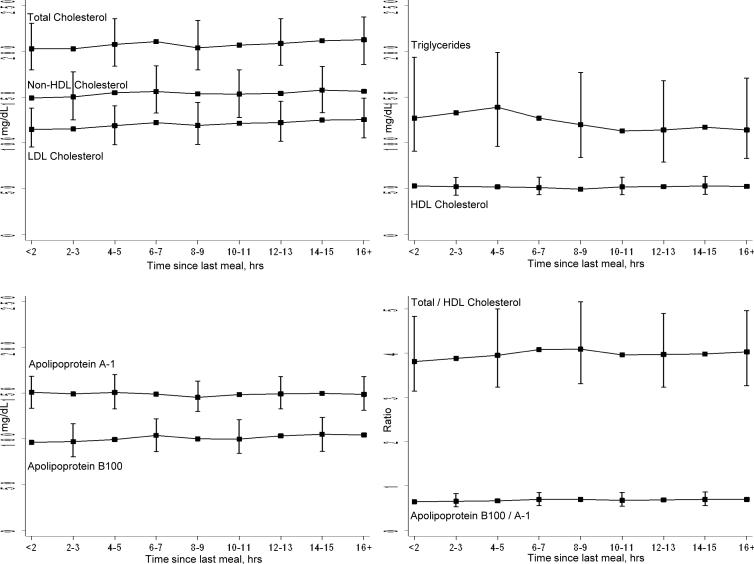
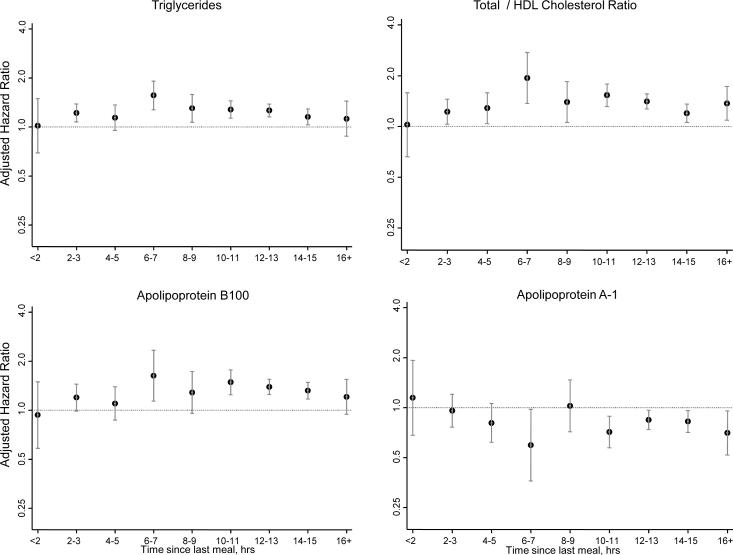
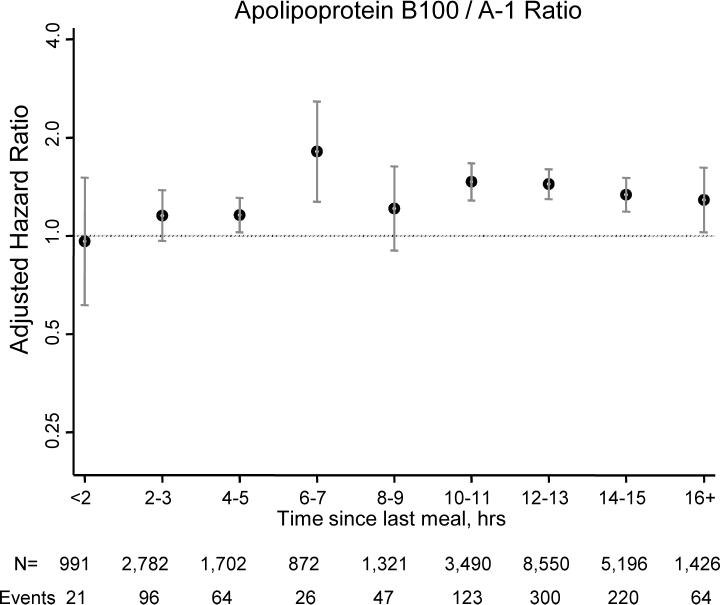
Next, to determine the effect of time since the last meal on concentrations of lipids and apolipoproteins, we plotted the median, 25th, and 75th percentile postprandial values by 2-hour intervals (Figure 1). Except for triglycerides, there were no substantial changes in the distributions of lipid and apolipoprotein concentrations as a function of time since the last meal. The highest levels of triglycerides were noted 4 to 5 hours postprandially, which was seen in women with HDL cholesterol <50 or ≥50 mg/dL. Figure 2 shows the adjusted hazard ratios (95% CIs) for CVD of each of the lipid and apolipoprotein concentrations (per 1-SD increments) depending on the time since the last meal before the blood draw. For total cholesterol, LDL cholesterol and non-HDL cholesterol, significant associations with CVD were noted only after at least 10 hours postprandially. By contrast, the strongest associations for the other lipids and apolipoproteins were noted 6 to 8 hours postprandially.
Figure 1.
Median and 25th to 75th percentile values for lipids and apolipoproteins as a function of time since the last meal.
Figure 2.
Hazard ratios and 95% confidence intervals (shown on a log-scale) for 1-standard deviation increment of lipids and apolipoproteins, adjusted for age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index.
DISCUSSION
In this prospective study of 26,330 initially healthy women, the concentrations of lipids and apolipoproteins differed minimally when measurements were performed on nonfasting compared with fasting blood, except for triglycerides which were higher when nonfasting. However, the associations with CVD were stronger for fasting compared with nonfasting measurements of total cholesterol, LDL cholesterol, apolipoprotein B100, non-HDL cholesterol, and the apolipoprotein B100/A-1 ratio. By contrast, the associations with CVD were similar for fasting and nonfasting HDL cholesterol, apolipoprotein A-1, and the total/HDL cholesterol ratio, and stronger for nonfasting triglycerides. These observations suggest that nonfasting blood draws may be highly effective and practical when limited to HDL cholesterol, total/HDL cholesterol ratio, and triglycerides. However, these data also suggest that a fasting sample is preferred if risk assessment is based on total cholesterol, LDL cholesterol, or non-HDL cholesterol.
Prior studies have found lower concentrations of LDL cholesterol postprandially, and higher triglycerides, with a similar magnitude of difference in our study compared with prior studies that used a typical non-high fat meal.6,15,16 Our finding that there was no significant difference between fasting and nonfasting measurements of HDL cholesterol and apolipoprotein A-1 is also consistent with other studies.17,18
However, to our knowledge, this is the first study that prospectively compares the association of a comprehensive panel of lipids and apolipoproteins with CVD depending on the time to last meal. While prior studies have evaluated the effect of food intake on lipid and apolipoprotein concentrations,6,16,17,19-22 the influence of postprandial time on the predictive value of lipids and apolipoproteins, other than triglycerides, is scarce. In a prior case-control report examining 683 postmenopausal healthy women that included some who were nonfasting, the results were not analyzed according to fasting status except for triglycerides, which showed similar prediction in the fasting or nonfasting state.23 The AMORIS study included a large proportion of women, with a third of participants who were nonfasting, but associations of lipids and apolipoproteins with fatal myocardial infarction were not examined according to fasting status.24 Other prospective studies that included a large number of nonfasting participants had data only on men and did not have comparisons with fasting lipids.25-27 Although differences between fasting and nonfasting concentrations of lipids and apolipoproteins, except for triglycerides, were small and clinically insignificant in our study, the strength of the association of various lipids and apolipoproteins with CVD differed by fasting/nonfasting status. Total cholesterol and LDL cholesterol showed the most marked differences in their predictive value, although different associations were also found for apolipoprotein B100, non-HDL cholesterol, and the apolipoprotein B100/A-1 ratio, all of which were stronger in the fasting state. This finding suggests that measuring a nonfasting measurement of total cholesterol or non-HDL cholesterol, which is currently believed to be acceptable, may need to be interpreted with caution.
As suggested by Figure 2, even stronger associations may be observed within 6 to 8 hours postprandially for lipids and apolipoproteins that represent part of the atherogenic dyslipidemia of the metabolic syndrome, such as HDL cholesterol and triglycerides, while longer durations of fasting (>10 to 12 hours) may be required for total cholesterol and LDL cholesterol. The underlying biological explanation for this is unclear, but may relate to the time course of postprandial triglyceride metabolism, since 4 to 8 hours is the time of peak triglycerides, and by 8 hours, triglyceride concentrations have returned to fasting concentrations in most individuals.28
There are several limitations of the present study. Time to last meal was self-reported, and we did not have both fasting and nonfasting measurements in the same individuals. Lipid measurements were only available once at baseline and results could not be corrected for potential regression dilution bias. We only had data on women, although no substantial differences were noted in women who were or were not taking hormones. Our study included healthcare professionals who were mostly white, apparently healthy, and recruited from a variety of geographic locations across the US; thus, it is unclear if our results would be applicable to other ethnic populations or men. Our statistical power was less in nonfasting than in fasting women. Finally, this was a primary prevention population and further studies are needed before the data can be extended to secondary prevention populations that are frequently treated with lipid lowering medications.
Strengths of the present study include the large number of healthy women participants with comprehensive measurements of a panel of lipids and apolipoproteins, including the direct measurement of standard lipids. Additionally, detailed information on cardiovascular risk factors was available, allowing for the control for potential confounding by these factors, such as the time of day of blood draw and hormone use. Finally, previous studies have not examined the influence of fasting status on the predictive ability of various lipids and lipoproteins, other than triglycerides, all of which was possible in this study due to the large number of participants in subgroups divided by time since the last meal.
In summary, this study demonstrates that HDL cholesterol, triglycerides, total/HDL cholesterol ratio, and apolipoprotein A-1 predict CVD when measured nonfasting. By contrast, total, LDL, and non-HDL cholesterol, in addition to apolipoprotein B100 and B100/A-1 ratio, may provide less useful CVD risk information when measured nonfasting, despite small changes in their concentrations. Guidelines for lipid screening may need to consider these differences.
Funding Sources
The Women's Health Study is supported by grants HL-43851 and CA-47988 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, and by grants from the Donald W. Reynolds Foundation, Leducq Foundation, and Doris Duke Charitable Foundation, with additional support from an Investigator-Initiated Studies Program from Merck. Dr Mora is supported by grants from the American Heart Association (0670007N), Sandra Daugherty Foundation, and Lerner Research Young Investigator Award. The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this manuscript.
Footnotes
Conflict of Interest Disclosures The authors report no conflicts.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Cats VM, Orth-Gomer K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145–155. doi: 10.1016/j.atherosclerosis.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Bachorik PS, Ross JW. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995;41:1414–1420. [PubMed] [Google Scholar]
- 4.Rifai N, Dufour DR, Cooper GR. Preanalytical variation in lipid, lipoprotein, and apolipoprotein testing. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of lipoprotein testing. 2nd ed. AACC Press; Washington, DC: 2000. pp. 161–187. [Google Scholar]
- 5.Craig SR, Amin RV, Russell DW, Paradise NF. Blood cholesterol screening influence of fasting state on cholesterol results and management decisions. J Gen Intern Med. 2000;15:395–399. doi: 10.1046/j.1525-1497.2000.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder LB, Bachorik PS, Finney CA, Moy TF, Becker DM. The effect of fasting status on the determination of low-density and high-density lipoprotein cholesterol. Am J Med. 1995;99:374–377. doi: 10.1016/s0002-9343(99)80184-3. [DOI] [PubMed] [Google Scholar]
- 7.Eberly LE, Stamler J, Neaton JD. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163:1077–1083. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
- 8.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 10.O'Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100:899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 12.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Hainline A, Karon J, Lippel K. Manual of Laboratory Operations: Lipid Research Clinics Program and Lipid and Lipoprotein Analysis. 2nd ed. Dept of Health and Human Services; Bethesda, Md.: 1982. [Google Scholar]
- 15.Dubois C, Beaumier G, Juhel C, Armand M, Portugal H, Pauli AM, Borel P, Latge C, Lairon D. Effects of graded amounts (0−50 g) of dietary fat on postprandial lipemia and lipoproteins in normolipidemic adults. Am J Clin Nutr. 1998;67:31–38. doi: 10.1093/ajcn/67.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Dubois C, Armand M, Mekki N, Portugal H, Pauli AM, Bernard PM, Lafont H, Lairon D. Effects of increasing amounts of dietary cholesterol on postprandial lipemia and lipoproteins in human subjects. J Lipid Res. 1994;35:1993–2007. [PubMed] [Google Scholar]
- 17.Cohn JS, McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456–2459. [PubMed] [Google Scholar]
- 18.Rifai N, Merrill JR, Holly RG. Postprandial effect of a high fat meal on plasma lipid, lipoprotein cholesterol and apolipoprotein measurements. Ann Clin Biochem. 1990;27:489–493. doi: 10.1177/000456329002700512. [DOI] [PubMed] [Google Scholar]
- 19.Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29:469–479. [PubMed] [Google Scholar]
- 20.Dubois C, Armand M, Azais-Braesco V, Portugal H, Pauli AM, Bernard PM, Latge C, Lafont H, Borel P, Lairon D. Effects of moderate amounts of emulsified dietary fat on postprandial lipemia and lipoproteins in normolipidemic adults. Am J Clin Nutr. 1994;60:374–382. doi: 10.1093/ajcn/60.3.374. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer EJ, Audelin MC, McNamara JR, Shah PK, Tayler T, Daly JA, Augustin JL, Seman LJ, Rubenstein JJ. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001;88:1129–1133. doi: 10.1016/s0002-9149(01)02047-1. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JA, Seman LJ, Ferrari A, Rubenstein JJ. Effects of atorvastatin on fasting and postprandial lipoprotein subclasses in coronary heart disease patients versus control subjects. Am J Cardiol. 2002;90:689–696. doi: 10.1016/s0002-9149(02)02591-2. [DOI] [PubMed] [Google Scholar]
- 23.Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, Stampfer MJ, Ma J. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110:2824–2830. doi: 10.1161/01.CIR.0000146339.57154.9B. [DOI] [PubMed] [Google Scholar]
- 24.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 25.Pocock SJ, Shaper AG, Phillips AN. Concentrations of high density lipoprotein cholesterol, triglycerides, and total cholesterol in ischaemic heart disease. BMJ. 1989;298:998–1002. doi: 10.1136/bmj.298.6679.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talmud PJ, Hawe E, Miller GJ, Humphries SE. Nonfasting apolipoprotein B and triglyceride levels as a useful predictor of coronary heart disease risk in middle-aged UK men. Arterioscler Thromb Vasc Biol. 2002;22:1918–1923. doi: 10.1161/01.atv.0000035521.22199.c7. [DOI] [PubMed] [Google Scholar]
- 27.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 28.Campos H, Khoo C, Sacks FM. Diurnal and acute patterns of postprandial apolipoprotein B-48 in VLDL, IDL, and LDL from normolipidemic humans. Atherosclerosis. 2005;181:345–351. doi: 10.1016/j.atherosclerosis.2004.12.045. [DOI] [PubMed] [Google Scholar]