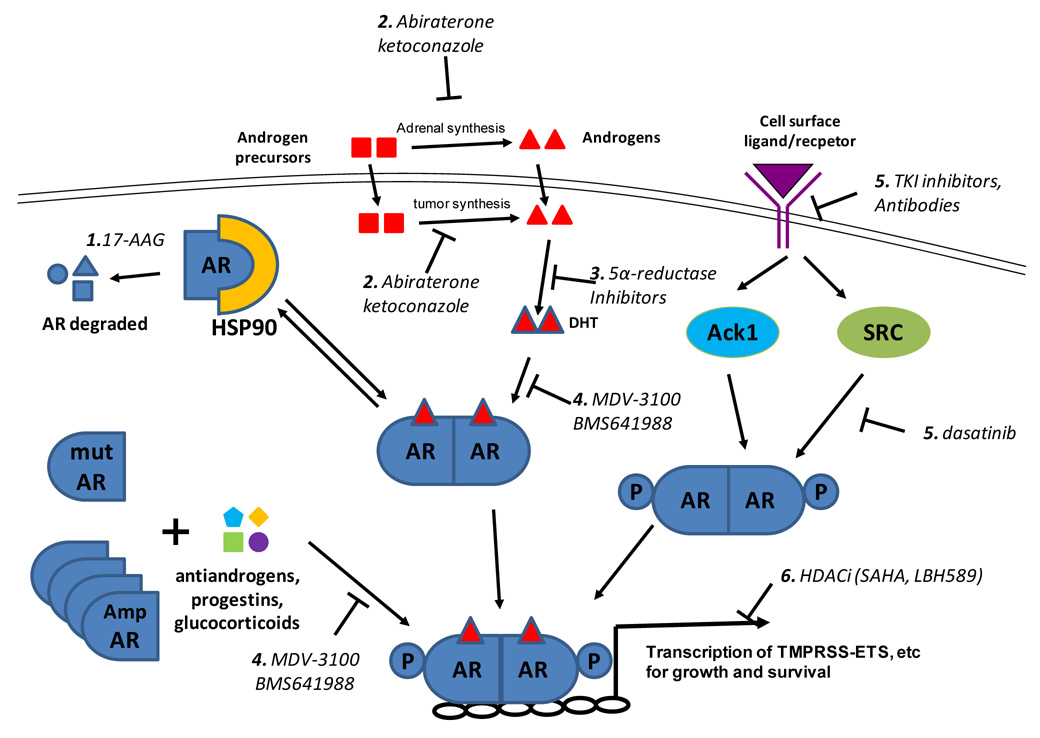
Figure 1.
Schematic of therapeutic targets of the AR pathway. 1) AR is bound to the molecular chaparonin HSP90 which prevents its degradation. HSP90 inhibitors, such as 17-AAG, cause AR degradation and decrease AR levels. 2) In men treated with GnRH agonists to shut down testicular androgen synthesis, residual serum androgens are synthesized by the adrenal glands. In additional, evidence suggests intratumoral androgen synthesis. Both can be inhibited by the non-specific p450 inhibitor ketoconazole and the 17-lyase inhibitor abiraterone. 3) Testosterone is converted to the more potent dihydrotestosterone (DHT) by 5α-reductase, which is inhibited by finasteride and dutasteride. 4) Ligands, such as DHT bind to AR and this is inhibited by antiandrogens such as bicalutamide and novel agents MDV-3100 and BMS641988. Mutation of AR as well as AR overexpression can convert endogenous steroids (e.g., progestins, estrogens, corticosteroids) and some antiandrogens into agonists. MDV-3100 was designed to suppress AR function even when AR is overexpressed. 5) Activation of receptor tyrosine kinases, in particular HER2, can lead to downstream AR activation. Two downstream kinases that directly phosphorylate AR on tyrosine are Ack1 and SRC. Other downstream pathways of receptor tyrosine kinases, including the AKT and MAP kinase pathways, are also implicated. Antibodies such as trastuzamab and pertuzumab and small molecular TKI inhibitors such as erlotinib and lapatinib target HER2. Dasatinib target SRC. 6) Proper transcription mediated by AR requires the proper chromatin state. HDAC inhibitors inhibit transcription of AR target genes by disruption of chromatin structure and inhibition of recruitment of coactivators and RNA polymerase II.