Abstract
Rationale:
Repeated amphetamine (AMPH) exposure is known to cause long-term changes in AMPH-induced locomotor behavior (i.e., sensitization) that are associated with similarly long-lasting changes in brain function. It is not clear, however, if such exposure produces long-lasting changes in a cognitive behavior that, in humans, is hypothesized to contribute to addiction.
Objectives:
To examine whether repeated AMPH exposure induces both locomotor sensitization and alters impulsive choice in a delay-discounting task.
Methods:
Adult, male Sprague-Dawley rats (n = 29) were pre-treated with 3.0 mg/kg AMPH or saline every other day for 20 days, and were then trained to lever press for small, immediately delivered food reinforcement or larger reinforcements delivered after delays. We subsequently assessed the effects of acute AMPH (0.1-2.0 mg/kg) on delay-discounting. Lastly, we tested for long-lasting effects of pre-treatment by giving an AMPH challenge (3.0 mg/kg) one week after the final delay-discounting session.
Results:
Repeated AMPH produced sensitization to the drug's stereotypy-inducing effects, but did not alter acquisition or baseline behavior in the delay-discounting task. Following acute AMPH, impulsive choice and other measures of delay-discounting were altered, but to a similar extent in both saline- and AMPH-pretreated groups. The AMPH challenge, given ∼3 months after the last pretreatment injection, revealed sensitization was still evident.
Conclusions:
Our results suggest that one behavioral consequence of repeated AMPH exposure – sensitization – does not overlap with another potential outcome – increased impulsivity. Furthermore, the neuroadaptations known to be associated with sensitization may be somewhat distinct from those that lead to changes in impulsive choice.
Keywords: delay-discounting, amphetamine, rat, decision making, stereotypy
Introduction
While the locomotor effects and cellular adaptations that result from repeated amphetamine (AMPH) exposure have been well characterized (e.g., Robinson and Becker 1986; Seiden et al. 1993; Robinson and Kolb 1997, 1999; Lu and Wolf 1999), the long-term consequences of such exposure on measures of cognitive function are not as well established. For instance, a number of clinical studies have demonstrated impairments in decision-making (Rogers et al. 1999; Ersche et al. 2006), concentration and attentional control (Deller and Sarter 1998; McKetin and Mattick 1998), and working memory (Ornstein et al. 2000). However, most of these impairments were observed shortly after cessation of drug administration (≤ three days), thereby leaving the possibility that they were influenced by acute withdrawal effects rather than more long-term, drug-induced neuroadaptations that might persist well beyond drug withdrawal. Also, the results observed in clinical studies may be influenced by the existence of polydrug abuse, varied duration and frequency of drug use and/or abstinence, and co-morbid psychiatric disorders (Khantzian 1985; Levin and Kleber 1995; Rosselli and Ardila 1996).
Recent studies using animal models, which offer an advantage of control over many of these variables, have provided further insights into the long-term effects of repeated AMPH exposure on cognition. For example, rats that exhibit behavioral sensitization have been shown to have deficits in tasks measuring attentional set-shifting (Fletcher et al. 2005), sustained visual attention (Fletcher et al. 2007), pre-pulse and latent inhibition (Tenn et al. 2003, 2005), and, in primates, working memory (Castner et al. 2005). Rats that self-administered AMPH over five different cycles of five days each, which were interspersed with a nine day drug-free period when they were tested in a five-choice serial reaction time task, exhibited deficits in visuospatial attention and no significant effects on perseverative (compulsive) or premature (impulsive) responding compared to yoked, saline-infused controls (Dalley et al. 2005). The changes in attentional performance recovered within five days of the withdrawal period, but reappeared following a two-month abstinence period and acute treatment with 0.8 mg/kg AMPH. Impulsive responding was attenuated by this dose of AMPH in the group of rats with AMPH self-administration experience.
Another task used to assess impulsive behavior in an operant reinforcement context is delay-discounting. In this paradigm, the apparent value of reward can be shifted by manipulating the time delay between a required response and the delivery of an expected reinforcement (Evenden and Ryan 1996). Specifically, small, immediately delivered reinforcements become more valuable than those that are large, but presented following a delay (Monterosso and Ainsle 1999). Thus, in this paradigm, choices of small reinforcements are taken to reflect impulsive behavior. Although increased impulsivity is considered to be characteristic of individuals who abuse drugs (e.g., Jentsch and Taylor 1999; Coffey et al. 2003; Kirby and Petry 2004; Heil et al. 2006; Hoffman et al. 2006; Verdejo-Garcia et al. 2007), there have been relatively few studies that have directly addressed the role of repeated psychostimulant exposure in altering impulsive choice behavior. Those that have (e.g., Richards et al. 1999; Paine et al. 2003; Roesch et al. 2007; Simon et al. 2007) suggest that chronic treatment with methamphetamine or cocaine increases impulsivity. However, a recent study by Winstanley et al. (2007) showed that repeated cocaine treatment did not alter baseline levels of impulsive choice. While this study was extensive, details about cocaine-induced locomotor activity (e.g., whether or not behavioral sensitization was observed) were not reported.
In the current study, we examined whether one behavioral consequence of repeated AMPH exposure—behavioral sensitization—overlaps with another potential outcome—impulsive choice. Because the delay-discounting task requires rats to be well-trained before responding becomes stable, any observed effects of AMPH pretreatment on task behavior are not likely to be due to disruptions caused by short-term withdrawal effects. After 40 daily sessions in the task, we examined whether acute treatment with saline (vehicle) or AMPH (0.1-2.0 mg/kg) just prior to delay-discounting test sessions altered subsequent task behavior. Lastly, rats in both the saline and AMPH pretreatment groups were given a challenge injection of AMPH (3.0 mg/kg) to determine if long-lasting changes in locomotor activity were evident. This challenge test occurred approximately 3 months after the last injection in the open-field.
Methods
Animals
Male Sprague-Dawley rats (n = 32), bred in our animal facility from stock rats obtained from Harlan (Indianapolis, IN), were housed individually starting at ∼2 months of age and were 3-4 months old (315-450 g) at the start of experiments. They were maintained on a 12:12 h light: dark cycle (lights on at 0800) with experimental sessions conducted between 0900 and 1800 h. Rats were handled five times for 15 min intervals prior to being used in experiments. With the exception of periods when rats were undergoing operant training and testing, food was available ad libitum. Water was always available ad libitum. All experimental procedures were approved by the IACUC at the University of Illinois, Urbana-Champaign and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
Apparatus
Locomotor activity was measured in an open-field chamber that consisted of a transparent, Plexiglas box (40.6 × 40.6 × 40.6 cm) surrounded by photobeams (Coulbourn Instruments; Allentown, PA). Each apparatus was connected to a computer operating software (TruScan, v. 2.01; Coulbourn Instruments) that recorded all horizontal and vertical beam breaks (100 ms sampling rate). The horizontal beam breaks, or coordinate changes, were converted into ambulatory distance (cm). The chambers were individually contained inside sound-attenuating cubicles (76 × 80 × 63 cm). Each cubicle contained a speaker (76 mm dia.) fixed to one wall, two ceiling-mounted white lights (4 W) for dim illumination, and a ceiling-mounted camera between the two lights.
Operant behavior was monitored in standard operant conditioning chambers (Coulbourn Instruments). One wall of the chamber contained two retractable levers that were positioned on either side of a centrally located food trough. Infrared detectors were used to monitor head entries into the food trough. White cue lights were located above each lever. A white houselight was located near the top of the chamber on the opposite wall.
Repeated saline or AMPH treatment
Prior to starting the 20-day, intermittent injection procedure, rats were first habituated to the open-field testing and injection procedures. Specifically, they were brought to the testing room and, following a 20-min acclimation period during which they remained in their home cages, were placed in the open-field arena for 30 min. They were then removed, injected with saline (1 ml/kg, i.p.), and returned to the arena for 60 min. On the next day (i.e., treatment day 1), the same procedure was repeated except half of the rats (n = 16) received saline and the remainder were given AMPH (3.0 mg/kg, i.p.; n = 16). Beginning on treatment day 2 and continuing every other day until treatment day 9, rats were brought to the laboratory, injected with saline or AMPH, and allowed to behave for 60 min in an acrylic tub (46 × 25 × 22 cm) lined with hardwood bedding. These tubs and bedding, which were identical to those used for the rat's home cage in the animal colony, were used as a means to provide multiple injection environments and thereby minimize context-dependent sensitization (Robinson and Becker 1986; Badiani and Robinson 2004). On treatment day 10, the open-field testing procedure used on treatment day 1 was repeated. Thus, rats received a total of 10 saline or AMPH injections over the course of 20 days, with automated (i.e., photobeam) activity measures obtained on the first and final injection days. The intermittent-dosing procedure was adapted from previous studies that showed impairments in object recognition memory (Bisagno et al. 2003; Belcher et al. 2006) and the development of behavioral sensitization (Robinson et al. 1985; Robinson and Becker 1986). Furthermore, pilot studies revealed that rats given AMPH using this procedure exhibited progressive increases in focused stereotypy that persisted for at least one month after treatment.
Delay-discounting behavior
Following their last test in the open-field arena, rats were placed on food restriction and maintained at 85% of their free-feeding weight. One week later, they were trained to respond on either of two independently available levers for a 45-mg food pellet (Bio-Serv; Frenchtown, NJ) on a fixed ratio schedule (FR1) of reinforcement during overnight sessions (2100 to 0900 hours). After they displayed approximately equal responding on both levers, rats were moved to the next training phase (1-h sessions, between 0900 and 1700 h). Trials began with levers retracted, and the food trough illuminated by a cue light. A nosepoke into the trough resulted in the extension of one randomly selected lever, with a subsequent lever press response reinforced by delivery of a food pellet. After 3-4 days of training at this stage, 14 saline pre-treated and 15 AMPH pre-treated rats began the final stage of training. Three rats (n = 2 and 1 from the saline and AMPH pre-treated groups, respectively) failed to complete the first two training stages within 15 sessions and were thus removed from the study. Two different cohorts of rats were trained on the task, with the second cohort responding for a different formulation of food pellet then the first (Bio-Serv products F0042 and F0021, respectively) at the end of training. This switch was made because of pellet feeder clogging problems that developed towards the end of the second cohort's training. Statistical analysis of behavioral performance within cohort two following this switch, and between cohorts one and two, revealed no significant change in task performance.
Training in the delay-discounting task was done in daily, 100 min sessions that consisted of five blocks of 12 trials. Each trial lasted 100 s and began with the illumination of the house light and cue light located in the food trough. Rats were required to nosepoke into the trough within 10 s, whereupon a single lever would be presented randomly and a response was reinforced with food pellet delivery. The amount of food delivered was pre-assigned to a lever, such that responses on one (e.g., left side lever) resulted in reinforcement that was large (four pellets) but delayed in delivery, and responses on the other (e.g., right side lever) resulted in an immediately delivered small reinforcement (one pellet). Assignment of reward magnitude to levers was counterbalanced across groups, but remained consistent for each rat. After a lever response, the house light turned off, the levers retracted, and the cue light above the lever was illuminated until food was delivered. If the rat either failed to nosepoke within 10 s or respond on the presented lever, the trial was recorded as an omission. On omission trials, the levers remained retracted and the chamber was returned to an intertrial interval (ITI) state until the beginning of the next trial. After completing or omitting the first two forced-choice trials (which served as exemplars for the each block), 10 free-choice trials were presented where both levers were extended. Delays for the delivery of large rewards increased with each block of trials (0, 10, 20, 40, and 60 seconds, respectively). Each animal participated in one session per day, for a minimum of 40 training sessions.
Following the last training session, the effects of AMPH on task behavior were assessed by administering rats injections of vehicle (saline; 1 ml/kg, i.p.) or AMPH (0.1, 0.3, 0.6, 1.0, and 2.0 mg/kg, i.p.) 5 min before they were placed in the operant conditioning chamber for a test session. Injections were given over eight consecutive daily sessions in the order of VDDVDDVD (V = vehicle; D = drug). The order of drug doses was chosen based on a Latin square design, with a particular order assigned to each rat randomly. The exception to this was the 2.0 mg/kg dose of AMPH, which was administered during the last session because pilot studies suggested this dose produced significant motor impairments that potentially could influence task performance in subsequent sessions.
Open-field behavior retest
After the final session in the delay-discounting task, rats were given access to food ad libitum. After one week, all rats were re-tested in the open-field with a challenge injection of 3.0 mg/kg AMPH. The same procedures used during the initial open-field behavioral tests were followed, including the time of day experiments were performed.
Drugs
d-Amphetamine sulfate was obtained from Sigma-Aldrich (St. Louis, MO). It was dissolved in sterile saline (0.9% NaCl), and dosages were calculated based on the weight of the salt. All injections were given at a volume of 1 ml/kg.
Data Analyses
Data were imported into a relational database (SQL Server 2005 Developer, Microsoft; Seattle, WA) and analyzed using SAS 9.1 (SAS Institute, Inc.; Cary, NC) or SigmaStat 3.5 (Systat Software, Inc.; San Jose, CA). All graphs show group means ± SEM. The effects of saline or AMPH on open-field behavior were analyzed using two-way, mixed factor ANOVA, with group as the between-subjects factor and treatment day as the repeated measure. Because 3.0 mg/kg AMPH tends to produce stereotyped patterns of behavior (i.e., repetitive head movements or sniffing), we also used a semi-quantitative scoring method (adapted from Gulley et al. 2004) to describe observed behavior. Raters blind to treatment conditions scored 30 sec segments of video taken every 5 min of the 60 min post-injection period. For each segment, the duration (0-30 sec) and intensity (1 – mild; 2 – moderate; 3 – intense) of focused stereotypy were recorded and these scores were then multiplied to give a single value (ranging from 0-90). These data were analyzed using three-way, mixed factor ANOVA with time and treatment day as within-subjects factors and group as the between-subjects factor. Follow-up analyses of significant interactions were performed with one- or two-way ANOVA and Holm-Sidak post-hoc tests.
For analysis of delay-discounting behavior, several measures were used. Choice behavior was represented as mean choice of the large reinforcer for free-choice trials only. The progression of task performance across sessions was assessed by obtaining these data during early acquisition (sessions 1-3), training midpoint (18-20), and stable baseline (sessions 41, 44, and 47). The stable baseline period encompassed the three sessions during which rats were given saline injections before they began the task. Data were analyzed with two-way repeated-measures ANOVA (treatment condition × delay). Data from tests of the acute effects of AMPH on task performance were analyzed using multifactorial ANOVA with treatment condition as a between-subjects factor and dose and delay as within-subjects factors. When appropriate, Holm-Sidak post-hoc tests were performed. Missing values were excluded from analysis. In cases where the assumption of sphericity was violated in repeated measures ANOVA, a multivariate analysis was used instead. In all tests, p values less than 0.05 were considered significant.
Results
Locomotor behavior
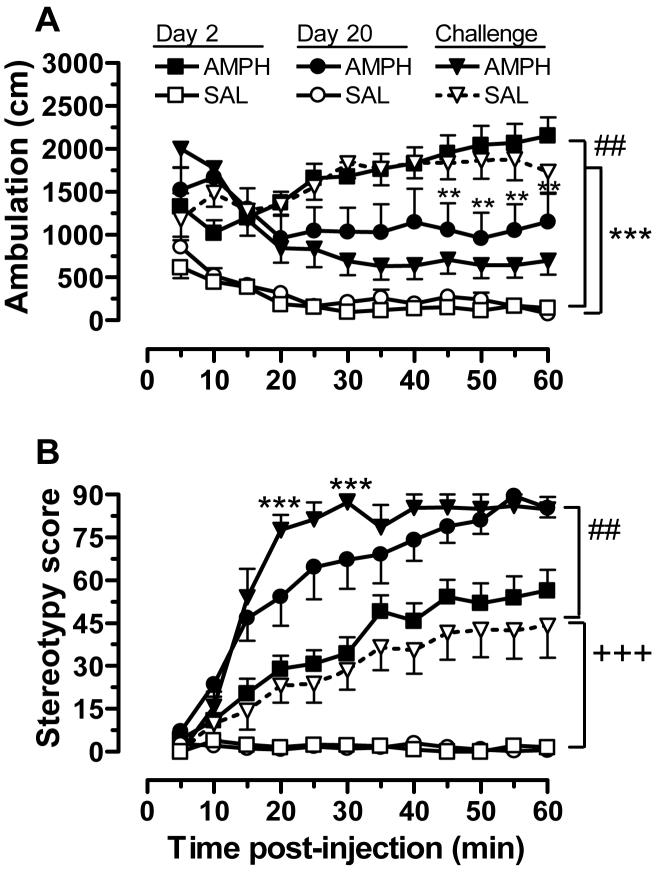
As expected, 3.0 mg/kg AMPH had robust effects on locomotor activity (Fig. 1). We observed significant main effects of group [F(1,936) = 174, p<.001], treatment day [F(2,936) = 61.5, p<.001], and time [F(11,936) = 2.58, p<.01] , along with a group × treatment day interaction [F(2,936) = 255, p<.001] and a three-way interaction [F(22,936) = 6.01, p<.001]. In the AMPH-pretreated group, the first drug exposure produced a significant increase in ambulation, compared to rats given saline (Day 2, Fig. 1A). After repeated AMPH treatment (10 injections; Day 20), however, ambulatory distance was significantly reduced compared to their first AMPH treatment day. This reduction, which was first apparent at 20 min following injection and became statistically significant at 45 min post injection, was due to the onset of focused stereotypy. As shown in Fig. 1B, the first exposure to AMPH led to increases in stereotypy that became maximal at ∼50 min following injection. After repeated exposure, rats in the AMPH pretreatment group exhibited maximal stereotypy by 30-min post injection. Mixed-factor ANOVA of these data revealed significant main effects for group [F(1,924) = 1420, p<.001], treatment day [F(2,924) = 217, p<.001], and time [F(11,924) = 44.8, p<.001], with a group × treatment day interaction [F(2,924) = 33.2, p<.001], and a three-way interaction [F(22,924) = 2.06, p<.001].
Figure 1.
Locomotor activity (ambulatory distance; A) or stereotypy rating (B) after acute AMPH or saline (Day 2), repeated AMPH or saline (Day 20), and the AMPH challenge given at the conclusion of the operant behavior phase of the study. AMPH was always given at a dose of 3 mg/kg (i.p.). Shown in the AMPH group are the data from 12 of 15 subjects tested because technical problems resulted in the loss of data from three subjects. **p<.01, Day 2 compared to Day 20 within the AMPH group; ##p<.01, between group comparison on Day 2; +++ p<.001, challenge compared to Day 2 and Day 20 within the SAL group; *** p<.001, challenge compared to Day 20 within the AMPH group; ###p<.001, between group comparison on challenge
One week after they finished testing in the delay-discounting task (see below), rats were re-tested in the open-field arena. During these tests, which occurred ∼ 3 months after their last open-field test session (i.e., treatment day 20), rats in both groups were injected with 3.0 mg/kg AMPH in the open-field arena. Rats in the saline pretreated group exhibited significant increases in both ambulation and stereotypy compared to their last saline test session (Fig. 1A and B). These effects were not significantly different from those observed in AMPH pretreated rats following their first AMPH injection, but it was different from drug-induced behavior in the AMPH pretreated group at re-test. In the AMPH-pretreated rats, there was no significant difference in ambulation at re-test compared to treatment day 20. Stereotypy was actually enhanced after the ∼ 3 month time lapse between injections of this AMPH dose, with scores reaching maximal values earlier in the 60 min post-injection time period (Fig. 1B).
Delay-discounting behavior
As shown in Fig. 2, prior exposure to AMPH did not alter delay-discounting behavior. There were no significant differences between saline and AMPH treatment groups during the early phase of task acquisition (sessions 1-3), at the midpoint of training (sessions 18-20), and during baseline (sessions 41, 44, and 47). Rats in both groups did exhibit a robust sensitivity to delay by reducing their responding on the large reward lever as the delay increased to a maximum of 60 s. The main effect of delay was significant at all three training stages [acquisition: F(4,24) = 4.33, p<.01; midpoint: F(4,24) = 22.0, p<.001; and baseline: F(4,24) = 73.5, p<.001]. As shown in Fig. 2A, the sensitivity to delay was apparent at the earliest stages of task acquisition, although it was not as pronounced during the 0-20 s delays compared to later sessions (Fig 2B and C). The number of trial omissions was relatively low, with rats in saline and AMPH pre-treatment groups omitting a total of 0.376 ± 0.114 and 0.196 ± 0.11 trials, respectively, across all delays during the midpoint sessions. This small group difference was not statistically significant, and neither were the small group differences in trial omissions at acquisition [saline: 1.18 ± 0.39; AMPH: 0.97 ± 0.38] and baseline [saline: 0.31 ± 0.09; AMPH: 0.16 ± 0.09].
Figure 2.
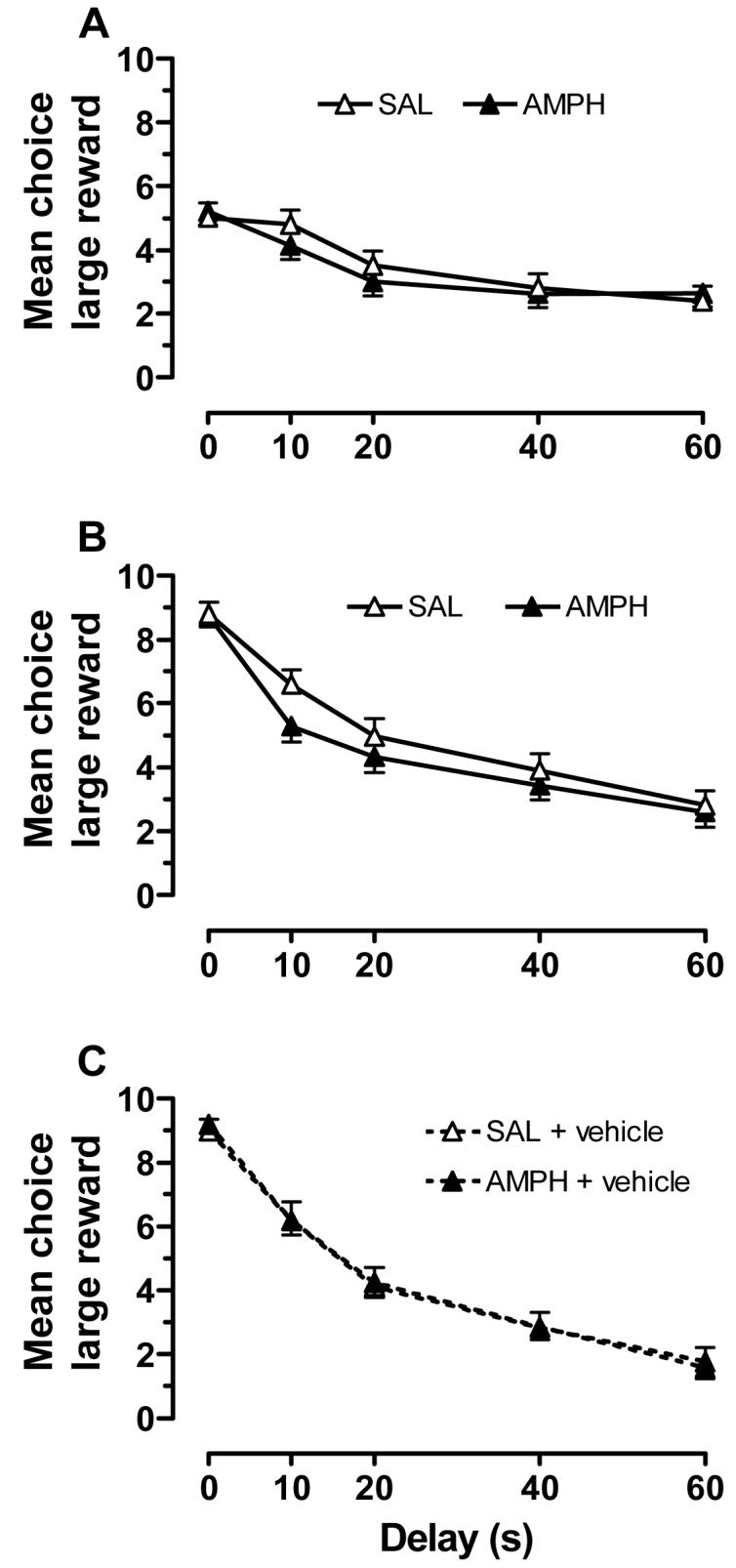
Choice behavior for rats in the saline (SAL; n=14) and AMPH (n=15) pretreatment groups for early acquisition (sessions 1-3; A), training midpoint (sessions 18-20; B), and at stable baseline (vehicle sessions 41, 44, and 47; C).
When AMPH was administered 5 min before the start of the task, it induced a dose-dependent change in choice behavior [dose: F(5,810) = 20.7, p<.001)] that varied as a function of delay [dose × delay: F(4,810) = 117, p<.001], but was not significantly different in saline and AMPH pretreated rats. Subsequent analysis of AMPH effects in the different treatment groups with two-way ANOVA and post-hoc analysis revealed that the 2.0 mg/kg AMPH significantly decreased choice of the large reward at the 0 and 10 s delays for saline pretreated rats and the 0, 10 and 20 s delays for those pretreated with AMPH (Fig. 3A-B). The number of trial omissions (Fig. 3C) and latencies to nosepoke or lever press (Fig. 4), in contrast, were increased over a wider range of AMPH doses and there were differential effects in the pretreatment groups. For omissions, we observed significant main effects of group [F(1,810) = 4.19, p<.05], dose [F(5,810) = 155, p<.001], and a group × dose interaction [F(5,810) = 50.3, p<.001]. Pairwise comparisons revealed that the 1.0 and 2.0 mg/kg doses were significantly different from vehicle, and the 2.0 mg/kg dose produced the most robust effect. The effects of the highest dose were most pronounced in the AMPH-pretreated group.
Figure 3.
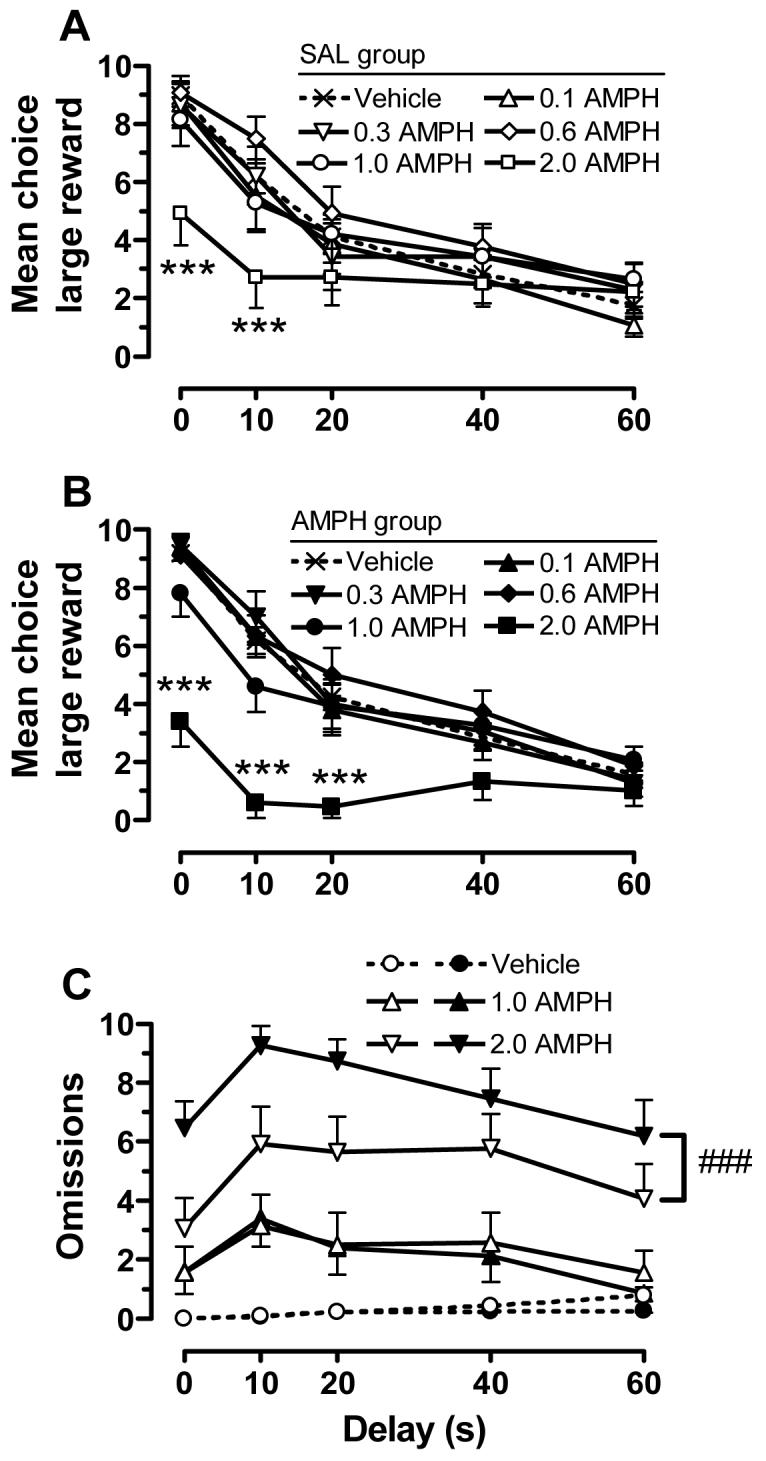
The effects of AMPH (0.1-2.0 mg/kg) on delay-discounting behavior in rats pretreated with saline or AMPH. Shown are the mean choice of the large reward for the saline (SAL; A) and AMPH (B) pretreated groups across delays. (C) Shown are the number of omissions as a function of delay for the SAL (open symbols) and AMPH (filled symbols) pretreatment groups. For enhanced presentation clarity, only the highest doses of AMPH are included. *** p<.001, compared to vehicle at the same delays; ###p<.001, collapsed across delay
Figure 4.
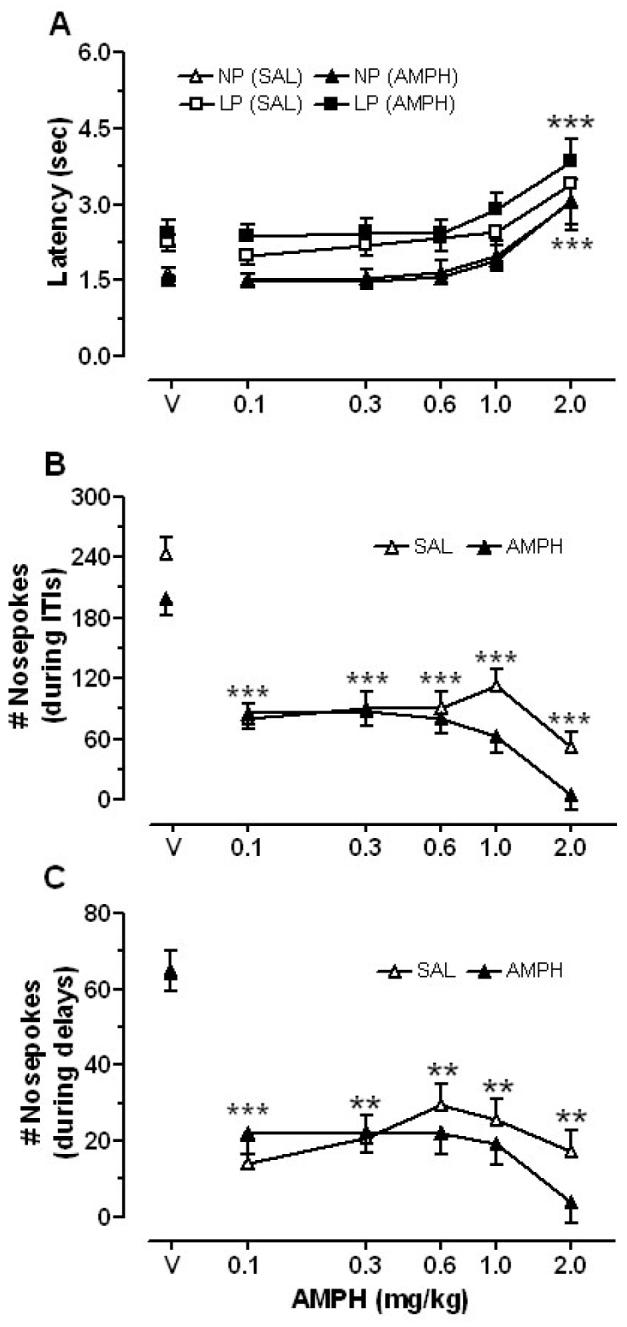
Dose-response curves showing the effects of AMPH (0.1-2.0 mg/kg) on (A) latency to nosepoke for the initiation of a trial (NP) and to choose a lever (LP), and (B) the total number of nosepoke responses during ITIs (B) and delays (C). **p<.01 and *** p<.001, compared to vehicle (V)
The latency to nosepoke for presentation of levers, as well as to respond on a lever, was increased by AMPH to a similar extent in both groups (Fig. 4A). There were significant main effects of dose for both measures [nosepoke latency: F(5,22) = 3.68, p<.01; lever press latency: F(5,16) = 3.53, p < .05], with the 2 mg/kg dose increasing latency to the greatest extent compared to vehicle injections. The number of nosepoke responses that rats made during periods when food pellets were not available—during ITIs and during delays following responses on the large reward lever—were decreased by all doses of AMPH (Fig. 4B and C). For nosepoke responses during ITIs and delays, only the main effects of dose were significant [ITIs: F(5,23) = 8.64, p<.001; delays: F(5,23) = 4.37, p<.01]. More specifically, all doses of AMPH reduced the number of nosepoke responses compared to vehicle sessions. This effect tended to be greater in AMPH-pretreated rats, but the main effect of group and the group × dose interaction were not significant. Also, the saline-pretreated group made more nosepoke responses during ITIs than the AMPH-pretreated group after vehicle and the highest tested doses of AMPH (Fig. 4B), although this effect was not statistically significant.
Discussion
Repeated exposure to 3.0 mg/kg AMPH produced robust changes in AMPH-induced locomotor activity in an open-field arena that was evident following 10 injections in 20 days. This treatment did not, however, alter baseline choice behavior in a delay discounting task. There were subtle effects on task performance when AMPH was given prior to the sessions, but these were likely due to motor disruptions produced by the higher doses and may be a reflection of the higher sensitivity to AMPH's locomotor effects that were observed in the open-field for this group. When rats in the AMPH pretreatment were given an AMPH challenge in the open-field after the conclusion of their final delay-discounting session—a procedure that occurred ∼3 months after their last injection in the open-field—the sensitization to AMPH's stereotypy-inducing effects was still evident and slightly enhanced. Rats in the saline pretreatment group exhibited AMPH-induced behavior that was not significantly different from that observed in the AMPH pretreatment group following their first drug injection. Taken together, these findings suggest that the long-lasting locomotor effects of repeated, intermittent AMPH exposure can be dissociated from effects on delay-discounting behavior. Furthermore, our results suggest that the neuroadaptations that resulted from repeated AMPH exposure, which are known to be associated with sensitization (Robinson and Berridge 2000), do not cause enduring deficits in the aspects of cognition that the delay-discounting task requires. It is not clear from the current study if the observed dissociation is specific to the dosing procedure used, but it is notable that previous studies using similar AMPH treatment schedules have reported a reduction in subsequent structural plasticity and forms of new learning (Kolb et al. 2003; Briand et al. 2005), as well as impairments in object recognition memory (Bisagno et al. 2003; Belcher et al. 2006).
With respect to the long-lasting sensitization that was observed in AMPH pretreated rats, it should be noted that these rats received an additional cumulative dose of 4.0 mg/kg AMPH across the 8 days of drug and vehicle testing in the delay-discounting task. The saline pre-treated group also had this same level of exposure, however, and their response to AMPH challenge was no different from that seen in AMPH pretreated rats after their first drug exposure. Thus, this additional exposure to AMPH is unlikely to account for the persistence of sensitization. Similar long-lasting changes in AMPH-induced behavior have been observed previously (Segal and Mandell 1974; Klawans and Margolin 1975; Kolta et al. 1985; Robinson and Becker 1986).
To our knowledge, no published studies have described the effects of chronic AMPH exposure on delay-discounting behavior in an animal model, but several have examined other psychostimulants (Richards et al. 1999; Paine et al. 2003; Roesch et al. 2007; Simon et al. 2007; Winstanley et al. 2007). While most of these reports suggested that repeated psychostimulant exposure increases impulsive decisions, the most extensive study to date (Winstanley et al. 2007) reported no baseline impairments in impulsive choice in rats exposed repeatedly to cocaine (15 mg/kg, i.p., for 21 days). Unfortunately, they did not report locomotor behavior, or more specifically, if behavioral sensitization was observed. As for the remaining studies, there are key factors that differ between each of them and ours. In the studies by Richards et al. (1999) and Paine et al. (2003), drug treatment began after training in the task was completed and injections were given following daily delay-discounting sessions. Interestingly, Paine et al. (2003) reported that increased impulsive choice in rats was only observed in association with behavioral sensitization (i.e., on day 7 of cocaine treatment; 15 mg/kg, 3 injections/day for 14 days), and choice behavior returned to baseline levels on subsequent days even though sensitization persisted or was enhanced. In a study by Simon et al. (2007) that utilized chronic treatment with a high dose of cocaine (30 mg/kg daily for 14 days), cue lights did not remain illuminated during the delay periods that occurred following responses on the large reward lever. The absence of cues has previously been shown to decrease rat's choices of the large reward lever (Cardinal et al. 2000), which is what the authors reported.
Simon et al. (2007) also observed a reduction in the number of nosepoke responses into the food trough during the delay periods prior to reward delivery. Interestingly, we did not observe this difference during vehicle sessions. At all tested doses of AMPH, however, we did find this effect. This is likely a separate construct from impulsive choice (Evenden 1999) and has been referred to as impulsive action. A few studies have addressed the influence of chronic AMPH exposure on impulsive action, and a task commonly used to test this characteristic is differential reinforcement of low rates of responding. Using this task, increased impulsive action was observed in AMPH-treated rats immediately following treatment (5.0 mg/kg for 9 days), but this only persisted for 9 days after the cessation of drug exposure (Peterson et al. 2003). Once again, these findings suggest that impulsive behavior is only transiently altered by repeated AMPH exposure.
In the present study, injections of 0.1-1.0 mg/kg AMPH had subtle effects on choice behavior in both pre-treatment groups. The 2.0 mg/kg dose increased the number of omissions and latencies to both nosepoke and lever press, with the AMPH-pretreated group exhibiting significantly more omissions than the saline-pretreated group. There are several reports indicating that acute AMPH treatment either dose-dependently reduces (Cardinal et al. 2000; deWit et al. 2002; Winstanley et al. 2003; vanGaalen et al. 2006), increases (Evenden and Ryan 1996; Cardinal et al. 2000), or has little to no effect (Uslaner and Robinson 2006) on impulsive choice in a delay-discounting paradigm. It is unclear why the results of these various studies are somewhat equivocal, but differences in baseline choice behavior have been shown to influence the effects of AMPH on delay discounting. For example, Winstanley et al. (2003) have suggested that AMPH decreases impulsive choice in rats that tend to have higher levels of impulsive choice before drug treatment. A recent study (Barbelivien et al. 2008), which systematically examined the interaction between baseline choice behavior and AMPH's effects on delay discounting, revealed that AMPH significantly decreased impulsive choice in rats with a “medium” basal level of impulsive choice and had no significant effect in those with either low or high baseline levels. In the present study, our sample was not sufficiently large to appropriately analyze sub-populations of rats with diverse choice behavior at baseline, but we did note a trend for AMPH to decrease impulsive choice most in rats with high to medium baseline levels, and an opposite effect in animals with low baseline levels of impulsive choice.
While current theories of drug addiction suggest that heightened impulsivity is a common characteristic of individuals who abuse drugs (Jentsch and Taylor 1999; Lyvers 2000; Goldstein and Volkow 2002; Bechara 2005), it is not clear whether this is a major contributing factor to the development of addiction or if it is caused by repeated drug exposure. Our study in rats suggests that increased impulsive choice is not an inevitable consequence of repeated, intermittent exposure to AMPH. Instead, it supports the notion that, at least in rats, a high degree of impulsivity is more likely a pre-existing trait that is potentially amplified by exposure to drugs (Bechara 2005; Dalley et al. 2007). Similarly, evidence from clinical studies (Ersche et al. 2008) suggests that repeated drug exposure does not lead to generalized effects: chronic cocaine use was associated with perseverative responding, whereas chronic amphetamine use was not. Taken together, these studies and our results underscore the importance of avoiding generalization between drugs of abuse and ensuing changes in behavior and the brain. Furthermore, our study suggests that the neuroadaptations that are known to be associated with the long-lasting expression of behavioral sensitization (Robinson and Berridge 2000) may not have wide-ranging consequences for other behaviors that are considered to be associated with, or characteristic of, addiction. Rather, there may be a unique or potentially overlapping set of drug-induced neuroadaptations that lead to heightened impulsivity.
Acknowledgements
We thank Alex Krueger, Acidalia Ortiz Rivera, and Sergei Pourmal for their technical assistance. This work was supported in part by a grant from the NIH (DA 019876), an Arnold O. Beckman Award from the University of Illinois, Urbana-Champaign, and a predoctoral fellowship to JJS (T32 NIH/HD007333).
References
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Barbelivien A, Billy E, Lazarus C, Kelche C, Majchrzak M. Rats with different profiles of impulsive choice behavior exhibit differences in responses to caffeine and d-amphetamine and in medial prefrontal cortex 5-HT utilization. Behav Brain Res. 2008;187:273–283. doi: 10.1016/j.bbr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O'Dell SJ, Marshall JF. A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav Brain Res. 2006;170:167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Chronic D-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacol Biochem Behav. 2003;74:859–867. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Briand LA, Robinson TE, Maren S. Enhancement of auditory fear conditioning after housing in a complex environment is attenuated by prior treatment with amphetamine. Learn Mem. 2005;12:553–556. doi: 10.1101/lm.95905. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Castner SA, Vosler PS, Goldman-Rakic PS. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 2005;57:743–751. doi: 10.1016/j.biopsych.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Berry D, Milstein JA, Laane K, Everitt BJ, Robbins TW. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology. 2005;30:525–537. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Deller T, Sarter M. Effects of repeated administration of amphetamine on behavioral vigilance: evidence for “sensitized” attentional impairments. Psychopharmacology (Berl) 1998;137:410–414. doi: 10.1007/s002130050637. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology (Berl) 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Reed JL, Kuwajima M, Rebec GV. Amphetamine-induced behavioral activation is associated with variable changes in basal ganglia output neurons recorded from awake, behaving rats. Brain Res. 2004;1012:108–118. doi: 10.1016/j.brainres.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Klawans HL, Margolin DI. Amphetamine-induced dopaminergic hypersensitivity in guinea pigs. Implications in psychosis and human movement disorders. Arch Gen Psychiatry. 1975;32:725–732. doi: 10.1001/archpsyc.1975.01760240053004. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolta MG, Shreve P, De Souza V, Uretsky NJ. Time course of the development of the enhanced behavioral and biochemical responses to amphetamine after pretreatment with amphetamine. Neuropharmacology. 1985;24:823–829. doi: 10.1016/0028-3908(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Levin FR, Kleber HD. Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry. 1995;2:246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- Lu W, Wolf ME. Repeated amphetamine administration alters AMPA receptor subunit expression in rat nucleus accumbens and medial prefrontal cortex. Synapse. 1999;32:119–131. doi: 10.1002/(SICI)1098-2396(199905)32:2<119::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: comparison with non-drug-using controls. Drug Alcohol Depend. 1998;50:181–184. doi: 10.1016/s0376-8716(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology (Berl) 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–147. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Impaired DRL 30 performance during amphetamine withdrawal. Behav Brain Res. 2003;143:101–108. doi: 10.1016/s0166-4328(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Moore CJ, Castaneda E, Mittleman G. Enduring enhancement in frontal cortex dopamine utilization in an animal model of amphetamine psychosis. Brain Res. 1985;343:374–377. doi: 10.1016/0006-8993(85)90760-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Ardila A. Cognitive effects of cocaine and polydrug abuse. J Clin Exp Neuropsychol. 1996;18:122–135. doi: 10.1080/01688639608408268. [DOI] [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenn CC, Fletcher PJ, Kapur S. Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality similar to that of schizophrenia. Schizophr Res. 2003;64:103–114. doi: 10.1016/s0920-9964(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Tenn CC, Kapur S, Fletcher PJ. Sensitization to amphetamine, but not phencyclidine, disrupts prepulse inhibition and latent inhibition. Psychopharmacology (Berl) 2005;180:366–376. doi: 10.1007/s00213-005-2253-z. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]