Figure 3. Site of ferric ion binding to CCK8SO4.
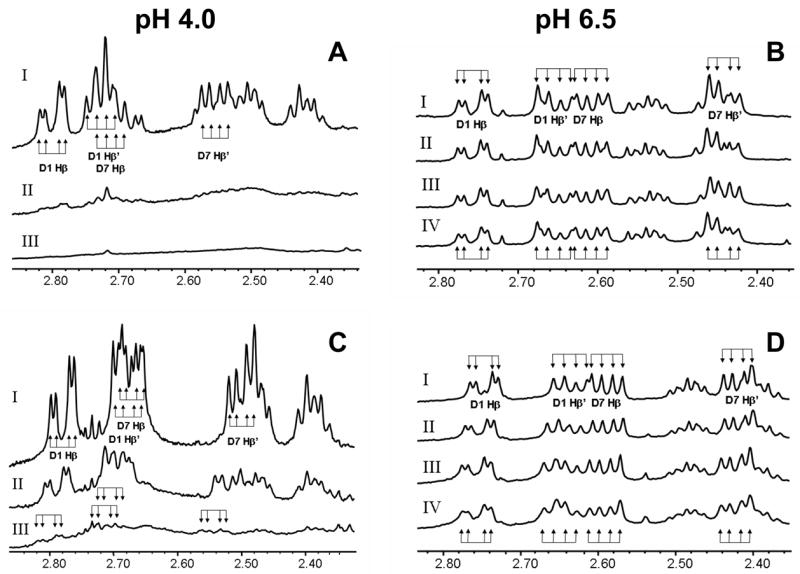
At pH 4.0 and 298 K addition to 50 μM CCK8PO4 (A) or CCK8SO4 (C) of 1 (spectrum II) or 3 mol/mol (spectrum III) of FeCl3 resulted in a substantial loss of signal intensity compared to the spectrum without ferric ions (spectrum I). These results indicate the formation of insoluble Fe3+-peptide complexes over the duration of the NMR experiment. For CCK8SO4 remaining in solution, shifts were observed in several resonances on addition of ferric ions. The largest change was 0.04 ppm for the Asp7 CHβ and CHβ′ resonances, while the change in the corresponding Asp1 peaks was only half as great. The Asp1 and Asp7 CHβ and CHβ′ resonances each appear as a doublet of doublets because of geminal coupling of each proton to its twin and vicinal coupling to the CHα proton. No such shifts were observed for CCK8PO4 remaining in solution. At pH 6.5 and 298 K addition to 140 μM CCK8PO4 (B) of 0, 1, 2 or 4 mol/mol of ferric ions (spectra I-IV, respectively) resulted in a slight broadening of all peaks in the spectrum. As the same amounts of ferric citrate were added to CCK8SO4 (D) there was a downfield shift in the Asp1 CHβ and CHβ′ resonances to a maximum of 0.02 ppm. Parallel but smaller changes in chemical shifts to a maximum of 0.01 ppm were observed for the Asp7 CHβ and CHβ′ resonances. The chemical shifts of other resonances were unaffected by addition of ferric citrate.
