Figure 4. Effect of tyrosine modification on receptor binding.
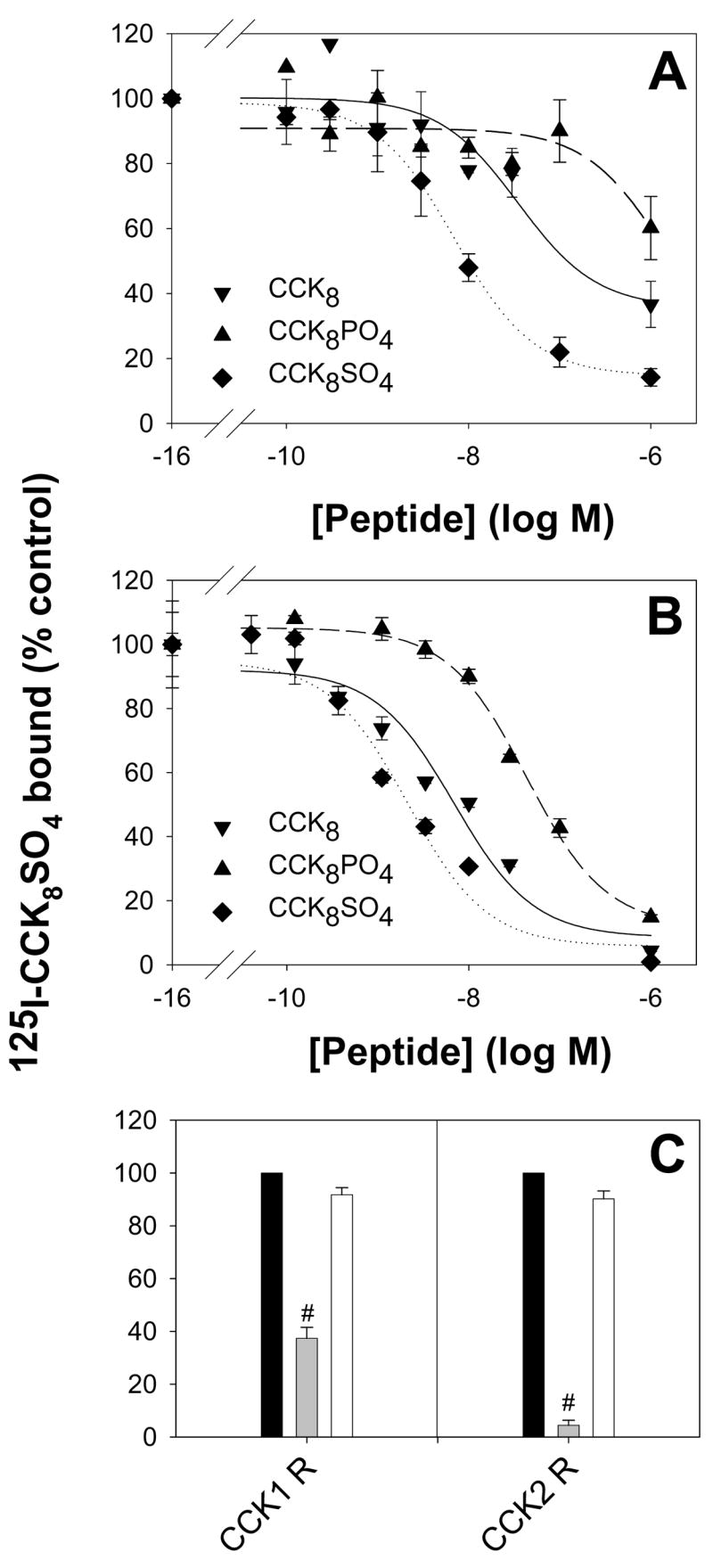
The ability of CCK8 (▼), CCK8SO4 (◆), or CCK8PO4 (▲), to compete with [125I]-Bolton and Hunter labelled-CCK8SO4 (150 pM, 100,000 cpm) for binding to the human CCK1 (A) or CCK2 (B) receptor on transiently transfected COS-7 cells was measured as described in Experimental. Points represent the mean data from at least three experiments, each in triplicate, and lines represent the best fit to a one site model. The IC50 values for the binding of CCK8, CCK8SO4 and CCK8PO4 to the CCK1 receptor were 35 ± 17 nM, 7.1 ± 0.7 nM and >1 μM, respectively, and the corresponding values for binding to the CCK2 receptor were 6.9 ± 2.0 nM, 2.1 ± 0.7 nM and 43 ± 7 nM, respectively. In contrast to the previously reported enhancement of binding on sulphation of CCK8, phosphorylation reduced the peptide’s affinity for both receptors. (C) The inability of the chelator desferrioxamine (DFO, 1 μM, white bars) to compete with [125I]-Bolton and Hunter labelled-CCK8SO4 for binding indicated that ferric ions were not essential for binding of CCK8 to the human CCK1 or CCK2 receptors. Unlabelled 1 μM CCK8SO4 (grey bars) served as a positive control. Values are the means ± S.E. of triplicates, expressed as a percentage of the value obtained in the absence of peptide competitor (black bars). Statistical significance was assessed by ANOVA (#, P < 0.001).
