Figure 5. Binding of calcium or zinc ions to phosphorylated CCK8 enhances subsequent ferric ion binding.
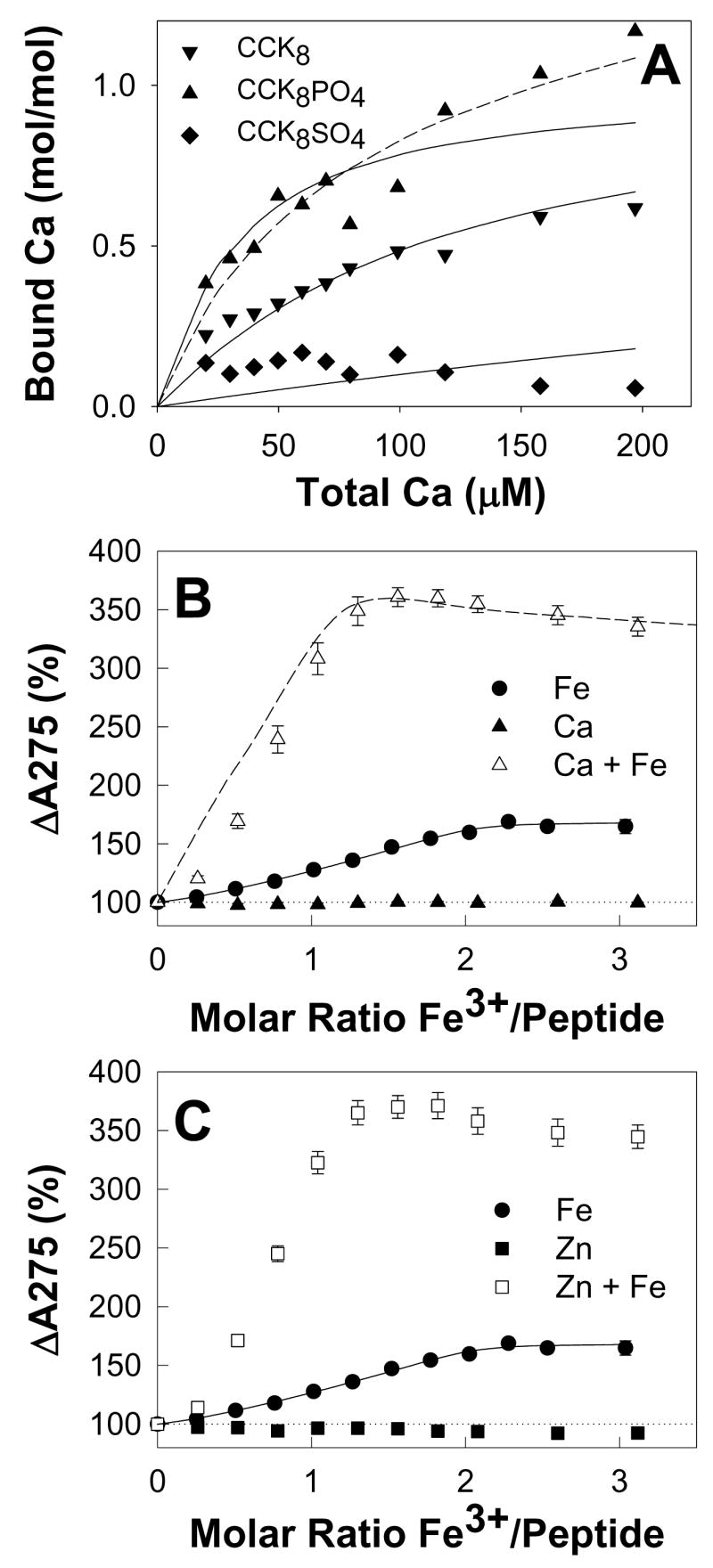
(A) The change in free [Ca2+] during addition of aliquots of calcium chloride to 44.6 μM CCK8 (▼),15.2 μM CCK8PO4 (▲) or 35.6 μM CCK8SO4 (◆) in 10 mM Na+ PIPES, pH 6.5, 100mM NaCl, 0.005% Tween 20, 0.4% DMSO, 293 K was measured with a calcium-selective electrode as described in Experimental. Points are means of at least three separate experiments; solid and dashed lines represent the best fits with the program BioEqs to one-site and two-site models, respectively. The CCK8PO4 data was better fitted by a two-site than a one-site model. Binding of Ca2+ to the first CCK8PO4 site (Kd = 46 μM) was tighter than binding to CCK8 (Kd = 83 μM), which in turn was at least 10-fold tighter than binding to CCK8SO4 (Kd = 870 μM). (B,C) Addition of aliquots of calcium chloride (B, ▲) or zinc sulphate (C,■) to 40 μM CCK8PO4 in the same buffer did not result in any increase in the absorption at 275 nm up to a molar ratio of 5. Subsequent addition of aliquots of ferric chloride to the solutions containing calcium (△) or zinc (□) resulted in a greater increase in absorption than that seen when ferric ions were added to CCK8PO4 in the absence of calcium or zinc ions (●). Points are means of at least three separate experiments; bars represent the SEM. The solid lines represent the best fit to a two-site ordered model with the program BioEqs, with the Kd and A275 values for ferric ions only given in Table 1. The data for ferric ions in the presence of calcium ions was simulated (dashed line) as described in Experimental with the equation for a competitive two-site ordered model and the Kd and A275 values given in Figure 6.
