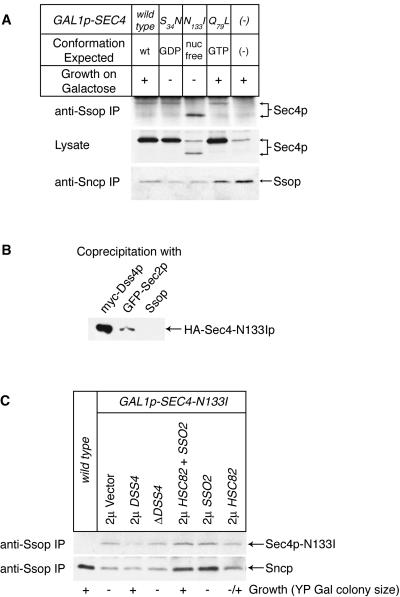
Figure 3.
Binding of Sec4-N133Ip to Ssop. (A) Binding of overexpressed Sec4 mutant proteins to Ssop. Cells overexpressing wild-type or mutant Sec4 proteins were grown to log phase in YP raffinose media and then shifted to YP galactose media for 6 h before homogenization. An immunoblot from the lysates was probed for Sec4 proteins; anti-Sso immunoprecipitates were probed for coprecipitating Sec4 proteins; and anti-Sncp immunoprecipitates were probed for coprecipitating Ssop. (B) Binding of HA-Sec4-N133Ip to myc-Dss4p, GFP-Sec2p, and Ssop. Cells expressing myc-Dss4p (NY1724) or GFP-Sec2p (NY1723) were mixed with HA-Sec4-N133Ip-expressing cells (NY1710) before homogenization. Myc-Dss4p was immunoprecipitated from the myc-Dss4p + HA-Sec4-N133Ip mixed lysate with anti-myc antibodies. The GFP-Sec2p from the GFP-Sec2p + HA-Sec4-N133Ip mixed lysate was immunoprecipitated with anti-GFP antibodies. For comparison, Ssop was also immunoprecipitated from the GFP-Sec2p + HA-Sec4-N133Ip mixed lysate. Coprecipitating HA-Sec4-N133Ip in the three immunoprecipitates was detected with anti-HA antibodies. HA-Sec4-N133Ip was detectable in the anti-Ssop immunoprecipitate on a longer exposure using the BLAZE detection system. (C) Differential effects in Sec4-N133Ip-overexpressing strains of 2μ DSS4 and SSO plasmids on growth and coprecipitation of Sec4-N133Ip and Sncp with Ssop. Strains were grown for 6 h in YP galactose media before lysis and immunoprecipitation with anti-Ssop antibodies. The immunoprecipitates were probed for coprecipitation of Sec4-N133Ip and Sncp. Suppression of the dominant-negative growth phenotype of Sec4-N133Ip overexpression was measured by observing colony sizes 3 d after streaking on YP galactose plates.