Table 1.
Optimization of chiral phosphonyl auxiliary.a
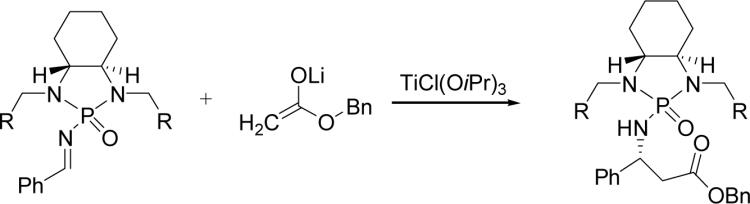 | ||||
|---|---|---|---|---|
| entry | imine | R | yield (%)b | drc |
| 1 | 1b | 1-nathphyl | 81 | 82:18 |
| 2 | 2 | Ph | 82 | 77:23 |
| 3 | 3 | 2-MeO-Ph | 80 | 72:28 |
| 4 | 4 | iPr | 60 | 76:24 |
Reaction condition: 0.1 mmol imine, 0.2 mmol ester, 0.22 mmol base, 2 equiv TiCl(OiPr)3, 2 mL solvent.
Isolated yield.
Determined by 31P NMR.
