DEFINITION & INTRODUCTION
Neuromuscular dysfunction of the defecation unit can lead to disordered or difficult defecation. Likewise, neuromuscular dysfunction of the colon may lead to slow transit constipation. Clearly, in many patients, there is an overlap, because colonic transit is delayed in two thirds of patients with difficult defecation (1,2). Preston and Lennard Jones (3) first described the association of paradoxical anal contraction during attempted defecation in patients with constipation and coined the term ‘anismus’. They felt that this condition was a spastic dysfunction of the anus, analogous to ‘vaginismus’. However, the term anismus implies a psychogenic etiology, which is not true although psychological dysfunction has been described in these patients. In the literature, a number of terms have been used to describe the constipation that is associated with anorectal dysfunction; which includes anismus (3), pelvic floor dyssynergia (4), obstructive defecation (1,5), paradoxical puborectalis contraction (6), and pelvic outlet obstruction (7,8) and spastic pelvic floor syndrome (9). Pelvic floor is a complex muscular apparatus that serves three important functions, namely, defecation, micturition and sexual function. All-encompassing terms such as ‘pelvic floor dyssynergia’ or “pelvic outlet obstruction” imply that this problem affects most of the pelvic floor, and possibly all of its functions. Although, some overlap has been described among patients with urinary obstruction and constipation (10), most constipated patients do not report sexual or urinary symptoms (11). Consequently, it misrepresents a functional disorder. Hence, these terms are not suitable. A consensus report from an international group of experts has recommended that the term dyssynergic defecation most aptly describes this form of constipation (12).
EPIDEMIOLOGY
The prevalence of chronic constipation (CC) varies from 2% to 28% (13). It is commonly encountered in primary care. Telephone interviews with 10,018 individuals, aged at least 18 years, produced an estimated prevalence of 14.7% (14). In a questionnaire survey of 5430 households across USA, functional constipation was reported by 3.6% of responders and difficult defecation by 13.8% (15). Because most patients do not seek health care, its prevalence has been underestimated (15).
Constipation is more common in women with an estimated female: male ratio of 2.2:1(15,16). Its prevalence increases with advancing age, particularly after age 65, with the elderly reporting more problems with straining and hard stools than infrequency (16). Its prevalence is twofold higher in African Americans (16), in those of lower socioeconomic status (annual income ≤ $20,000), (16), and in nursing home residents (16). Pregnancy is also associated with higher prevalence of constipation, but no differences were seen between the first and the last trimester (17).
Economic and Social Impact
Chronic constipation has a significant impact on the utilization of healthcare resources, including the cost of inpatient and outpatient care, laboratory tests, and diagnostic procedures (18). In a recent study of 76,854 patients enrolled in Medical program, the total healthcare expenditure for patients with constipation over a 15-month period was $18,891,008, with an average cost of $246 per patient (19). Approximately, 0.6% of patients were hospitalized with an average cost of $2993/admission (19). In another study, expenditure for constipation was estimated at $235 million/year with 55% incurred from inpatient, 23% from emergency department and 22% from outpatient care (20).
Psychological Distress, Abuse & Impact on Quality of Life
Constipation is associated with increased psychological distress. Several studies have shown higher prevalence for anxiety, depression, obsessive compulsiveness, psychoticism and somatization(21,22). Furthermore, paranoid ideation and hostility subscores were higher in patients with dyssynergia than STC or healthy controls, providing evidence for significant psychological distress, more so in dyssynergics than STC patients (22).
Sexual abuse was reported by 22% – 48% of subjects, mostly women, whereas physical abuse was reported by 31%–74% of constipated subjects (23,24). Another study found greater incidence of sexual abuse in women with pelvic floor dyssynergia (24). Also, patients with abuse were more likely to seek healthcare and report feelings of incomplete evacuation or urge to defecate, but did not demonstrate rectal hypersensitivity (25).
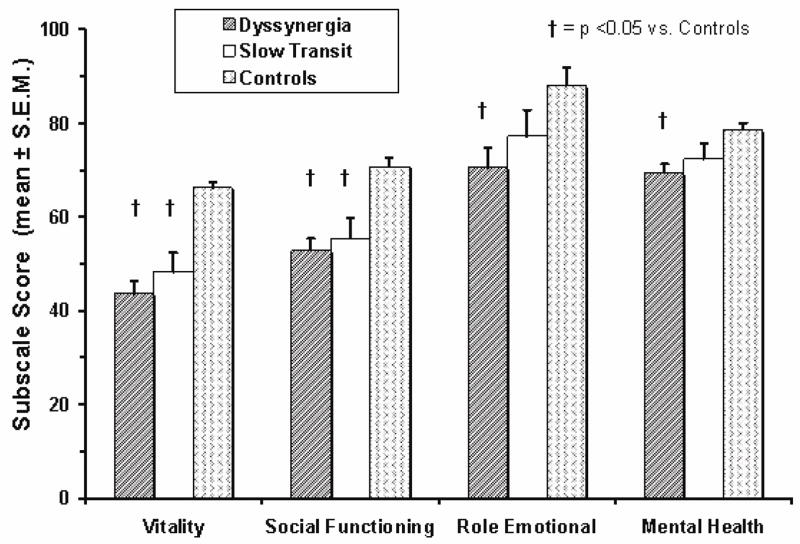
Patients with CC also showed significant impairment of health-related quality of life (Fig. 1) (22,26). Some domains were more affected in dyssynergics than STC (22), suggesting that dyssynergia is associated with greater impact on quality of life. Also, psychological distress and lower quality of life were strongly correlated suggesting that these dysfunctions have synergistic effects on bowel function (22).
Fig. 1.
Impact of chronic constipation on quality of life in patients with dyssynergic defecation, slow transit constipation and healthy controls. (Ref. 22).
ETIOLOGY/PATHOPHYSIOLOGY
Origin
How, when and why an individual develops dyssynergic defecation is unclear. Our prospective survey of 100 patients with dyssynergic suggested that the problem began during childhood in 31% of patients, after a particular event, such as, pregnancy, trauma or back injury in 29% of patients, and no identifiable precipitating cause in 40% of patients (23). Thus, 2/3rds acquire this condition during adulthood. In this group, 17% reported a history of sexual abuse, 43% the passage of hard stools frequently and 16 % intermittently. Thus, excessive straining to expel hard stools, over time, may also lead to dyssynergic defecation.
Pathophysiology
Earlier studies suggested that paradoxical anal contraction or involuntary anal spasm (anismus) during defecation may cause this problem (3). Consequently, myectomy of the anal sphincter was performed (8), but only 10 to 30% of patients improved (27). Likewise, paralyzing the anal sphincter muscle with Botulinum toxin injections produced minimal improvement (28). Hence, either spasm or inability to relax the external anal sphincter is unlikely to be the sole mechanism that leads to dyssynergic defecation.
A prospective study (1), showed that most patients with dyssynergic defecation demonstrate the inability to coordinate the abdominal, rectoanal and pelvic floor muscles to facilitate defecation. This failure of rectoanal coordination consists of either impaired rectal contraction (61%), paradoxical anal contraction (78%) or inadequate anal relaxation. Thus, incoordination or dyssynergia of the muscles that are involved in defecation is primarily responsible for this condition. In addition, 50–60% of patients also demonstrate an impaired rectal sensation (1).
CLINICAL FEATURES
Patients with dyssynergic defecation present with a variety of bowel symptoms. Often, patients do not volunteer or misrepresent their symptoms. For example, patients do not readily admit that they use digital maneuvers to disimpact stool or splint their vagina to facilitate defecation. However, by establishing a trustworthy relationship or through the help of symptom questionnaires or stool diaries, it may be possible to identify the precise nature of their bowel dysfunction. It is essential to determine this because only then can one approach this problem more rationally. In a prospective study, excessive straining was reported by 85%, a feeling of incomplete evacuation by 75%, the passage of hard stools by 65%, and a stool frequency of less than 3 bowel movements per week by 62% of patients (23). In addition, 66% of patients used digital maneuvers to facilitate defecation. In another study of 134 patients, two or fewer stools/week, laxative dependence and constipation since childhood was associated with slow transit constipation, whereas backache, heartburn, anorectal surgery and a lower prevalence of normal stool frequency was reported by patients with pelvic floor dysfunction (29). They concluded that symptoms are good predictors of transit time but poor predictors of pelvic floor dysfunction. A study of 190 constipated patients showed that stool frequency alone was of little value in constipation (30). In contrast, a sense of obstruction/digital evacuation was specific but not sensitive for disordered dysfunction. They also concluded that symptoms alone cannot differentiate between the pathophysiologic sub groups that lead to constipation (30).
Differential diagnosis, include many structural or functional abnormalities that may also lead to an evacuation disorder such as rectocele, hypertensive anal sphincter, hemorrhoids, anal fissure, anorectal neoplasia, rectal prolapse and proctitis. These conditions can be readily identified through appropriate testing. In contrast, functional evacuation disorders are less well recognized and poorly managed. These include dyssynergic defecation, excessive perineal descent and mucosal intussusception. Also, paradoxical anal contraction has been described in patients after pouch reconstruction (31). Many patients with the solitary rectal ulcer syndrome also exhibit dyssynergic defecation (32).
Patients with defecation disorders have several psychological abnormalities (22). This includes problems such as obsessive compulsive disorder- where the patient believes that having a bowel movement everyday or sometimes several times per day are the norm. A deviation from this process compels the individual to use laxatives, enemas, suppositories or any other means to achieve an unphysiological pattern of bowel movement. Others have phobia for stool impaction. This particularly affects children, who then learn quickly to exploit minor disturbance in defecation for seeking attention (33). The problem may also be driven by psychosocial issues such as inter-parental or parental/child conflicts or sibling rivalry. It has been shown that parental disattachment during childhood can lead to bowel dysfunction in adult life (34). Finally, patients with bulimia or anorexia nervosa and others with a history of physical or sexual abuse may also develop profound defecation problems (35).
DIAGNOSTIC PROCEDURES
General Issues
The first step in making a diagnosis of dyssynergic defecation is to exclude an underlying metabolic or pathologic disorder. Slow transit constipation may co-exist with dyssynergic defecation (1,36) and hence, an assessment of colonic motor function and transit is useful. An evaluation of the distal colonic mucosa through flexible sigmoidoscopy may provide evidence for chronic laxative use, may reveal melanosis coli or other mucosal lesions such as solitary ulcer syndrome, inflammation or malignancy.
Digital Rectal Examination
A careful perianal and digital rectal examination is not only important but often the most revealing part of clinical evaluation. Anorectal inspection can detect skin excoriation, skin tags, anal fissures or hemorrhoids. Assessment of perineal sensation and anocutaneous reflex by gently stroking the perianal skin with a cotton bud (Q-tip) or blunt needle in all four quadrants will elicit reflex contraction of the external anal sphincter. If this is absent, a neuropathy should be suspected. Digital rectal examination may reveal a stricture, spasm, tenderness, mass, blood or stool. If stool is present, its consistency should be noted and the patient should be asked if they were aware of its presence. A lack of awareness of stool in the rectum may suggest rectal hyposensitivity. It is useful to assess the resting and squeeze tone of the anal sphincter and puborectalis muscle by asking the subject to squeeze. More importantly, the subject should be asked to push and bear down as if to defecate. During this maneuver, the examiner should perceive relaxation of the external anal sphincter and/or the puborectalis muscle, together with perineal descent. A hand placed on the abdomen can gauge the abdominal push effort. An absence of these normal findings should raise the index of suspicion for an evacuation disorder such as dyssynergic defecation (37). Digital rectal examination has a high sensitivity for identifying dyssynergia (37). Even though digital rectal examination is a useful clinical tool, there is a lack of knowledge on how to perform a comprehensive evaluation. A survey of 256 final year medical students revealed that 17% had never performed a digital rectal exam and 48% were unsure of giving an opinion based on their findings (38). Thus, a concerted effort is needed to improve the training of digital rectal examination.
Anorectal Manometry
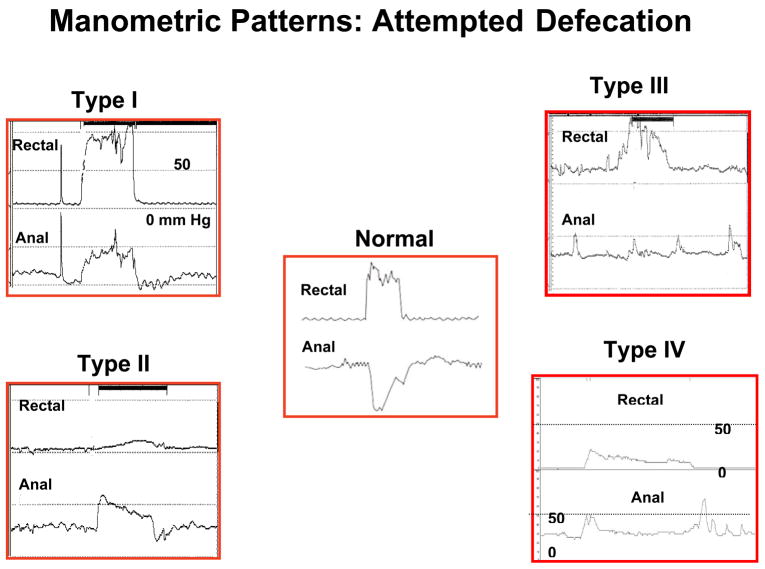
This test provides a comprehensive assessment of pressure activity in the rectum and anal sphincter region together with an assessment of rectal sensation, rectoanal reflexes and rectal compliance (39,40,41). Anorectal manometry is essential for a diagnosis of dyssynergic defecation (39,41). First, it excludes the possibility of Hirschsprungs disease. Normally, when a balloon is distended in the rectum there is reflex relaxation of the internal anal sphincter that is mediated by the myenteric plexus. This reflex response is absent in patients with Hirschsprungs disease. Second, it helps to detect abnormalities during attempted defecation. Normally, when a subject bears down or attempts to defecate, there is a rise in rectal pressure, which is synchronized with a relaxation of the external anal sphincter (Fig. 2). This maneuver is under voluntary control and is primarily a learned response. The inability to perform this coordinated movement represents the chief pathophysiologic abnormality in patients with dyssynergic defecation. This may either be due to impaired rectal contraction, paradoxical anal contraction or impaired anal relaxation or a combination of these mechanisms. Based on these features at least four types of dyssynergia can be recognized (Fig. 2).
Fig. 2.
This series reveals manometric patterns that are commonly seen during attempted defecation in a normal healthy individual (central panel) and in patients with dyssynergic defecation. They were obtained after placing a multisensor solid state manometry catheter into the rectum: changes from a single sensor in the rectum and one from the anal canal are shown. In the center panel, it can be seen that the subject can generate a good pushing force (increase in intra rectal pressure) and simultaneously relax the anal sphincterl. This is a normal pattern of defecation. In contrast, patients with dyssynergic defecation exhibit one of four abnormal patterns of defecation. In type I dyssenergia, the subject can generate an adequate propulsive force (rise in intra rectal pressure ≥40 mmHg) along with paradoxical increase in anal sphincter pressure. In type II dyssynergia, the subject is unable to generate an adequate propulsive force; additionally there is paradoxical anal contraction. In type III dyssynergia, the subject can generate an adequate propulsive force but there is either absent relaxation (a flat line) or incomplete (≤20%) relaxation of anal sphincter. In type IV dyssynergia, the subject is unable to generate an adequate propulsive force together with an absent or incomplete relaxation of anal sphincter.
| Type 1: | Here, the patient can generate an adequate pushing force, (rise in intra abdominal pressure) along with a paradoxical increase in anal sphincter pressure (Fig. 2). |
| Type 2: | Here, the patient is unable to generate an adequate pushing force (no increase in intrarectal pressure) but can exhibit a paradoxical anal contraction (Fig. 2). |
| Type 3: | Here, the patient can generate an adequate pushing force (increase in intrarectal pressure) but, either has absent or incomplete (<20%) sphincter relaxation (i.e. no decrease in anal sphincter pressure) (Fig. 2). |
| Type 4: | The patient is unable to generate an adequate pushing force and demonstrates an absent or incomplete anal sphincter relaxation (Fig. 2). |
In addition to the motor abnormalities described above, sensory dysfunction may also be present. Both the first sensation and the threshold for a desire to defecate may be higher in about 60% of patients with dyssynergic defecation (1,5). This may also be associated with increased rectal compliance. It must be noted that during attempted defecation some subjects may not produce a normal relaxation largely due to the laboratory conditions (42,43). Hence, this pattern alone should not be considered diagnostic of dyssynergic defecation (see diagnostic criteria below).
By observing the attempts to defecate, it is possible to identify the recording that most closely resembles a normal pattern of defecation. This recording can then be used to measure the intra rectal pressure, the anal residual pressure and the percentage of anal relaxation. The residual anal pressure is defined as the difference between the baseline pressure and the lowest (residual) pressure within the anal canal when the subject is bearing down (1,40). The % of anal relaxation is calculated using the formula, % anal relaxation = anal relaxation pressure/anal resting pressure × 100. From these measurements it is possible to derive an index of the forces required to perform defecation – the defecation Index. The defecation index may serve as a simple and useful quantitative measure of the rectoanal coordination during defecation (1,40).
Balloon Expulsion Test
In this test, either a silicone filled stool-like device such as the fecom (44) or a 4 cm. long balloon filled with 50 cc of warm water is placed in the rectum (40,44). A stop watch is started and the attendant leaves the room to provide privacy for the patient during balloon expulsion. The patient is then asked to expel the device and to stop the clock. Most normal subjects can expel a stool-like device within one minute, failing which dyssynergic defecation should be suspected. Although quite specific for dyssynergia, its sensitivity is approximately 50%.
Defecography
Defecography is commonly performed by placing approximately 150 ml of barium paste into the patient’s rectum. The patient is asked to sit on a special commode adjacent to a video fluoroscopic imaging system. The patient is instructed to squeeze or to evacuate the barium, and simultaneously the structural and functional changes of the anorectum are monitored by fluoroscopy and recorded on a videotape. This test provides useful information about anatomic and functional changes. In patients with dyssynergic defecation, the test may reveal poor activation of levator muscles, prolonged retention of contrast material or inability to expel the barium or the absence of a stripping wave in the rectum. However, patients often find this test embarrassing. Also, the type and consistency of barium paste varies considerably among different centers (45). Because of these inherent deficiencies, this test should be regarded as an adjunct to clinical and manometric assessment of anorectal function and should not be relied upon as a sole test for assessing an evacuation disorder (45).
Diagnostic Criteria for Dyssynergic Defecation
Most published studies have used arbitrary or symptomatic diagnostic criteria. For example, paradoxical anal contraction has been considered to be a sign-quo-non for dyssynergic defecation. However, we found that one study reported during attempted defecation, five patients showed either no change in the anal resting pressure or an insignificant (less than 20%) decrease (1); but, all of these patients failed to expel a balloon and also had greater than 50% retention of barium material during defecography. Similarly, two other patients were able to expel the balloon but had paradoxical anal contraction. Others have found that paradoxical anal contraction or insufficient (< 20%) decrease in anal EMG activity, colonic transit and defecographic abnormalities were not exclusively seen in patients with difficult defecation and none of the three tests showed significant differences in the prevalence of anismus between patients with or without slow transit constipation (46). Similarly, about 2/3rds of patients with constipation had objective evidence of delayed transit or pelvic floor dysfunction and no single test could reliably identify any of the pathophysiologic groups of constipation (47). Because a given patient may exhibit some but not all of the aforementioned dysfunctions, it is important to use more than one yardstick to diagnose this condition. In a prospective study, the presence of constipation symptom together with dyssynergic pattern of defecation and at least one additional abnormal test such as prolonged balloon expulsion time or prolonged colonic transit or excessive barium retention with defecography had a high diagnostic field of identifying dyssynergic defecation (48). In order to diagnose this condition, it has been proposed that a patient must satisfy both the symptomatic and the physiologic criteria set forth in Table 1.
Table 1.
|
TREATMENT
The treatment of a patient with dyssynergic defecation consists of:
Standard treatment for constipation
Specific treatment i.e. neuromuscular training or biofeedback therapy
Other measures including, botulinum toxin injection, myectomy or ileostomy.
Standard Treatment
This should consist of a detailed assessment and correction of coexisting issues such as avoiding constipating medications, increasing fiber and fluid intake and exercise activity. In a recent study, dietary instructions had little impact on fiber or nutrient intake in patients with dyssynergia, but about a third of patients were consuming a low fiber diet, and in this group their fiber intake increased (49). In addition, patients should receive instructions regarding timed toilet training and laxatives. Timed toilet training consists of educating the patient to attempt a bowel movement at least twice a day, usually 30 minutes after meals and to strain for no more than 5 minutes. During attempted defecation, they must be instructed to push at a level of 5 to 7, assuming level 10 as their maximum effort of straining. They should be encouraged to capitalize on intrinsic physiologic mechanisms that stimulate the colon, such as after waking (50,51) and after a meal (51). It is important to emphasize that stool impaction should be prevented at all costs. Patients should be advised to refrain from manual maneuvers such as digital disimpaction of stools.
Fiber Supplements
Organic polymers such as bran or psyllium have the ability to hold extra water and often resist digestion and absorption in the upper gut. However, there is no evidence that constipated patients in general consume less fiber than nonconstipated patients, and in fact studies show similar levels of fiber intake (49,52). Furthermore, constipated patients with slow transit or pelvic floor dysfunction respond poorly to dietary supplementation with 30 grams of fiber per day, whereas those without an underlying motility disorder improved (53). A fiber intake of 20 to 30 grams per day is optimal. Recently, both the ACG task force (54) and a systematic review (55) concluded that psyllium, a natural fiber supplement increases stool frequency and gave this compound a grade B recommendation, but there was insufficient data to make a recommendation for the synthetic polysaccharide methylcellulose, or calcium polycarbophil or bran in patients with constipation.
Pharmacologic Approaches
In one report, $821 million was spent on over-the-counter laxatives in USA (56). Several types of laxatives are available.
Stool Softeners
Sodium and calcium docusate compounds (Colace®, SURFAK®) are anionic surfactants that lower the surface tension of stool and facilitate the mixing of aqueous and fatty substances and also stimulate intestinal fluid secretion. There are four randomized controlled trials that have compared stool softeners with either placebo or other laxatives. The sample sizes were small and the data were conflicting (55). Consequently, these compounds were afforded a Grade B recommendation (54,55).
Stimulant Laxatives
This group consists of anthraquinones (senna, casacara sagrada, danthron and casanthronol), diphenylmethane derivatives (bisacodyl, sodium picosulphate) and ricinoleic acid (castor oil). Stimulant laxatives affect electrolyte transport across the intestinal mucosa and enhance colonic transport and motility; and usually work within several hours of administration.
Their long-term safety has not been established. Four randomized controlled trials were identified but none of them were placebo-controlled, the study design was of low quality and hence, a grade B recommendation was given (55).
Osmotic Laxatives
Osmotic laxatives include saline laxatives (salts of magnesium, phosphate, and sulfate), poorly absorbed synthetic disaccharides such as lactulose, sugar alcohols such as sorbitol or mannitol, and an inert polymer, polyethylene glycol (PEG-3350). This group includes ions or molecules that are not well absorbed by the intestine and require retention of water by the intestinal lumen to maintain osmotic balance with plasma.
Polyethylene glycol, PEG 3350 (Miralax®; Braintree Labs, Braintree, MA; glycolax®) is a large polymer that is poorly absorbed, metabolically inert and is not degraded by bacteria. It has been widely used as lavage solutions in preparation for colonoscopy. There are at least 8 placebo-controlled randomized control trials of PEG compounds and two randomized control trials comparing PEG with lactulose. PEG was superior to placebo in increasing stool frequency and stool consistency (55). A recent study reported relief of constipation in 52% of patients on PEG-3350 versus 11% of patients on placebo (57).
Chloride Channel Activators
Chloride channels are located in the apical and serosal membranes of the enterocyte and they facilitate chloride transport (58). There are four subtypes (59). Lubiprostone is a gastrointestinal-targeted bicyclic fatty acid that selectively activates Type 2 chloride channels. In a randomized controlled trial involving 237 patients, lubiprostone 24 μg twice daily for 28 days was more effective than placebo in increasing the number of spontaneous bowel movements, decreasing straining, improving stool consistency, and relieving symptoms of chronic constipation (60). Long-term studies show that the compound is efficacious and safe (61).
Miscellaneous & Emerging Therapies
Colchicine, a plant alkaloid used to treat gout and misoprostol, a prostaglandin analogue used to treat peptic disorders induce diarrhea as a side effect. Consequently, they have been tried in patients with chronic constipation (62,63). Another compound linaclotide, a guanylate cyclase agonist has been shown to accelerate gut transit in healthy subjects and in female patients with IBS-C (64).
Specific Treatment
Biofeedback Therapy
The goal of neuromuscular training using biofeedback techniques is to restore a normal pattern of defecation. Neuromuscular training or biofeedback therapy is an instrument-based learning process that is based on “operant conditioning” techniques. The governing principal is that any behavior-be it a complex maneuver such as eating or a simple task such as muscle contraction-when reinforced its likelihood of being repeated and perfected increases several fold. In patients with dyssynergic defecation, the goal of neuromuscular training is two-fold (2,65,66).
To correct the dyssynergia in coordination of the abdominal, rectal and anal sphincter muscles in order to achieve a normal and complete evacuation (Fig. 3).
To enhance rectal sensory perception in patients with impaired rectal sensation.
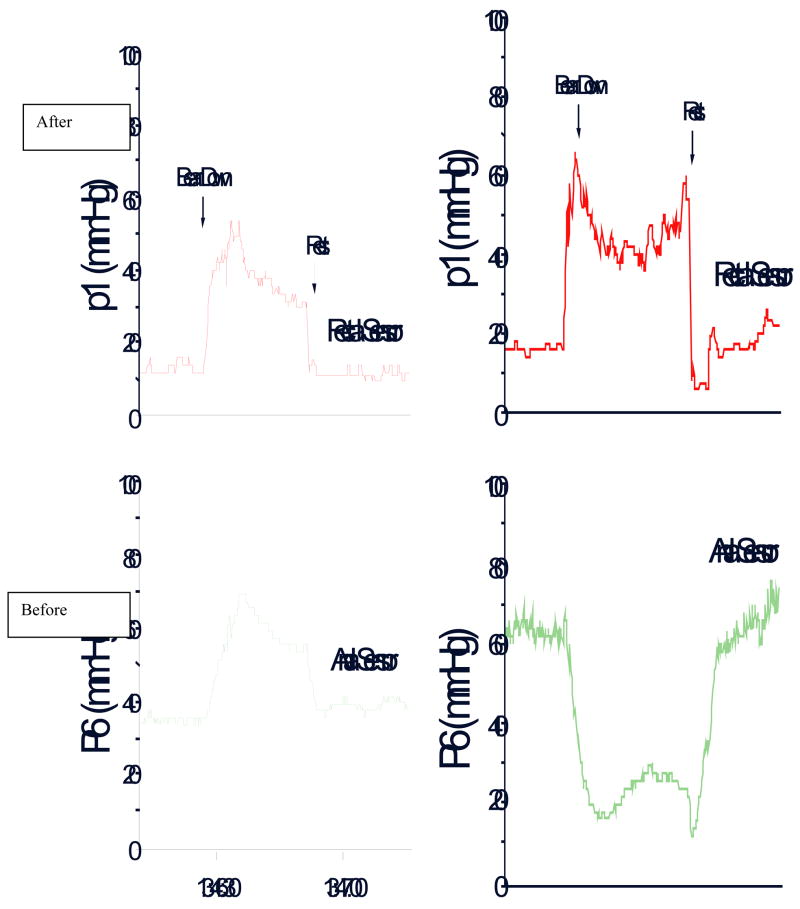
Fig. 3.
The rectal and anal pressure changes, and manometric patterns in a patient with constipation and dyssynergic defecation, before and after biofeedback therapy.
(i) Improve or Correct Dyssynergia
This training consists of improving the abdominal push effort (diaphragmatic muscle training) together with manometric guided pelvic floor relaxation followed by simulated defecation training: An outline of the protocol used at Iowa for biofeedback training is shown in Table 2.
Table 2.
Biofeedback Therapy for Constipation - Iowa Protocol
|
Rectoanal coordination
The purpose of this training is to produce a coordinated defecatory movement that consists of an adequate abdominal push effort as reflected by a rise in intra-rectal pressure on the manometric tracing that is synchronized with relaxation of the pelvic floor and anal canal as depicted by a decrease in anal sphincter pressure (Fig. 3). To facilitate this training, ideally the subject should be seated on a commode with the manometry probe in situ. After correcting the patient’s posture (for example, keeping the legs apart as opposed to keeping them together) and the sitting angle at which he/she will attempt the defecation maneuver, i.e. leaning forward, the subject is asked to take a good diaphragmatic breath and to push and bear down as if to defecate (2,65,66). The subject is encouraged to watch the monitor while performing this maneuver. The subject’s posture and breathing techniques are continuously monitored and corrected. The visual display of the pressure changes in the rectum and anal canal on the monitor provides instant feedback to the subject regarding their performance and helps them to understand and learn quickly [Fig. 3]. At least 10–15 maneuvers are performed.
Next, the balloon in the rectum is distended with 60 cc of air to provide the subject with a sensation of rectal fullness or desire to defecate. As soon as the subject experiences this desire, he/she is then encouraged to push and attempt defecation while observing the pressure changes in the rectum and anal canal on the display monitor. Once again the breathing and postural techniques are corrected. The maneuvers are repeated approximately 5 to 10 times. During the attempted defecation, the patient is instructed to titrate the degree of abdominal push and the anal relaxatory effort and in particular not to push excessively, as this is often counterproductive and leads to voluntary withholding. After each attempt, the balloon is deflated and re-inflated prior to the next attempt. After completion of this maneuver, the balloon is fully deflated and the probe is removed. If using an EMG device, the goal is to teach the subject to either reduce the amplitude of electrical wave forms on the monitor or to decrease the intensity of sound signals (67).
Simulated Defecation Training
The goal of this training is to teach the subject to expel an artificial stool in the laboratory using the correct technique. This maneuver is performed by placing a 50 ml water-filled balloon in the rectum or by using an artificial stool such as Fecom (65,68). After placement of balloon in the left lateral position, the subject is asked to sit on a commode and to attempt defecation. While the subject attempts to pass the balloon, assistance is provided, and the subject is taught to relax the pelvic floor muscles and to correct the posture and breathing techniques. If the subject is unable to expel the balloon, gentle traction is applied to the balloon to supplement the patient’s efforts. Gradually, the subject learns how to coordinate the defecation maneuver and to expel the balloon.
(ii)Sensory Training
The goal of this training is to improve the thresholds for rectal sensory perception and to promote better awareness for stooling (65,68). This is performed by intermittent inflation of the balloon in the rectum. The primary objective is to teach the subject to perceive a particular volume of balloon distention but with the same intensity as they had previously experienced with a larger volume of balloon distention. The first step here is to progressively inflate the balloon until the subject experiences an urge to defecate. This threshold volume is noted. After deflation, the balloon is re-inflated to the same volume and the maneuver is repeated two or three times to educate the subject and to trigger appropriate rectal sensations. Thereafter, with each subsequent inflation, the balloon volume is decreased in a stepwise manner by about 10%. During each distention, the subject is encouraged to observe the monitor and to note the pressure changes in the rectum and simultaneously pay close attention to the sensation they are experiencing in the rectum. They are encouraged to use the visual cues for volumes that are either not readily perceived or only faintly perceived. If the patient fails to perceive a particular volume or reports a significant change in the intensity of perception, the balloon inflation is repeated after a 5 second warning either by using the same volume or by using the previously perceived (higher) volume. Thus, by repeated inflations and deflations and through a process of trial and error, by the end of each session, newer thresholds for rectal perception are established.
Duration and Frequency of Training
The number of neuromuscular training sessions and the length of each training session should be customized for each patient depending on their individual needs. Typically, each training session takes one hour. Patients are usually asked to visit the motility laboratory once in two weeks. On average, 4 to 6 training sessions are required (65,68). At the outset, it is difficult to predict how many sessions a particular subject will need. After completion of neuromuscular training, periodic reinforcements at six weeks, three months, six months and twelve months may provide additional benefit, and also improve the long term outcome of these patients (65), but its role has not been examined.
Devices and Techniques for Biofeedback
Because neuromuscular training is an instrument-based learning technique, several devices and methods are available, and newer techniques continue to evolve. These include manometric-based biofeedback treatment with a solid-state manometry system, EMG biofeedback, balloon defecation training and home training devices (66). The solid-state manometry probe with microtransducers and a balloon is ideally suited for biofeedback therapy. Here, the transducers that are located in the rectum and anal canal provide a visual display of pressure activity throughout the anorectum. This display provides visual feedback to the subject. If required, surface EMG electrodes can be incorporated on the probe to provide both visual and auditory feedback. Sensory training can also be performed with the same probe. Thus, this system can serve as a comprehensive device for neuromuscular training.
Alternatively, an EMG biofeedback system that consists of a surface EMG electrode that is mounted on a probe or affixed to the surface of the external anal sphincter muscle can be used (67,69). These electrodes pick up EMG signals from the surface of the anal sphincter muscle and these are in turn displayed on the monitor. This provides instant visual feedback. The pitch of the auditory signals can be used to provide instant feedback regarding the changes in electrical activity of the anal sphincter. Such feedback responses can augment the learning process by helping the patient to titrate the defecation effort.
Home training devices largely use an EMG home trainer or silicon probe device attached to a hand-held monitor with an illuminated liquid crystal display (LCD). The pressure or electrical activity of the patient’s sphincter responses can be displayed on a simple gauge or on a strip chart recorder or on a color LCD display and these are used to provide visual feedback for the subject.
Efficacy of Biofeedback Therapy
The symptomatic improvement rate has varied between 44% up to 100% in several uncontrolled clinical trials (70). However, when interpreting the outcome of these studies, one should exercise caution because the end point for a successful treatment has been poorly defined, the duration of follow up and the selection of patients has been quite variable. However, in the last few years, several randomized controlled trials of adults with dyssynergic defecation have been reported and are summarized in Table 3. There are significant methodological differences between the studies and in the recruitment criteria as well as in the end points and outcomes. However, all of these studies have concluded that biofeedback therapy is superior to controlled treatment approaches such as diet, exercise and laxatives (68) or use of polyethylene glycol (67), diazepam/placebo (69), balloon defecation therapy (72) or sham feedback therapy (68).
Table 3.
– Summary of the randomized controlled trials of biofeedback therapy for Dyssynergic Defecation
| Chiaironi et al (71) | Rao et al (68) | Chiaironi et al (67) | Heymen et al (69) | |
|---|---|---|---|---|
| Trial Design | Biofeedback vs PEG 14.6 gms | Biofeedback vs. standard vs. sham biofeedback | Biofeedback for slow transit vs Dyssynergia | Biofeedback vs Diazepam 5 mg vs placebo |
| Subjects and Randomization | 104 women
54 biofeedback 55 polyethylene glycol |
77 (69 women)
1:1:1 distribution |
52 (49 women)
34 dyssynergia 12 slow transit 6 mixed |
84 (71 women)
30 biofeedback 30 diazepam 24 placebo |
| Duration & Number of biofeedback sessions | 3 months & 1 year, 5 weekly, 30 minute training sessions performed by physician investigator | 3 months, Biweekly, one hour, maximum of six sessions over three months, performed by biofeedback nurse therapist | 5 weekly 30 minute training sessions, performed by physician investigator | 6 bi-weekly, one hour sessions |
| Primary outcomes | Global Improvement of symptoms
Worse=0 No improvement=1 Mild=2 Fair=3 Major improvement=4 |
1. Presence of dyssynergia
2. Balloon expulsion time 3. Number of complete spontaneous bowel movements 4. Global satisfaction |
Symptom improvement
None=1 Mild=2 Fair=3 Major=4 |
Global Symptom relief |
| Dyssynergia corrected or symptoms improved | 79.6% reported major improvement at 6 and 12 months
81.5% reported major improvement at 24 months |
Dyssynergia corrected at 3 months in 79% with biofeedback vs 4% sham and 6% in Standard group; CSBM= Biofeedback group vs Sham or Standard, p<0.05 | 71 % with dyssynergia and 8% with slow transit alone reported fair improvement in symptoms | 70% improved with biofeedback compared to 38% with placebo and 30 % with diazepam |
| Conclusions | Biofeedback was superior to laxatives | Biofeedback was superior to sham feedback and standard therapy | Biofeedback benefits dyssynergia and not slow transit constipation | Biofeedback is superior to placebo and diazepam |
Biofeedback therapy is a labor intensive and multi-disciplinary approach but has no adverse effects. However, it is only offered in a few centers. In order to treat the vast number of constipated patients in the community, a home based, self-training program is essential. A large statewide study that employed home trainers demonstrated the feasibility of home training, but the efficacy of therapy was not compared and objective parameters of anorectal function were not assessed (73). In another European study, significant improvement was reported in most subjects receiving home therapy (7), but there was no control group.
Other Measures for Treating Dyssynergic Defecation
Injection of botulinum toxin into the anal sphincter has been tried with mixed results (28). In both studies there was some improvement in less than one half of patients but troublesome incontinence occurred in one study (74). The surgical aspects of managing dyssynergic defecation are discussed in Chapter 7.
SUMMARY
Constipation due to dyssynergic defecation is common and affects up to one half of patients with this disorder. This acquired behavioral problem is due to the inability to coordinate the abdominal and pelvic floor muscles to evacuate stools. Today, it is possible to diagnose this problem through history, prospective stool diaries, and anorectal physiological tests. Randomized controlled trails have now established that biofeedback therapy is not only efficacious but superior to other modalities and that the symptom improvement is due a change in underlying pathophysiology. Development of user friendly approaches to biofeedback therapy and use of home biofeedback programs will significantly enhance the adoption of this treatment by gastroenterologists and colorectal surgeons. Improved reimbursement for this proven and relatively inexpensive treatment will carry a significant impact on the problem, and this could translate into significant improvement of symptoms for patients with this disorder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rao SSC, Welcher K, Leistikow J. Obstructive Defecation. A failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–50. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 2.Rao SSC. Dyssynergic Defecation. Gastroenterol Clin North Am. 2001;30:97–114. doi: 10.1016/s0889-8553(05)70169-2. [DOI] [PubMed] [Google Scholar]
- 3.Preston DM, Lennard-Jones J. Anismus in chronic constipation. Dig Dis Sci. 1985;30:413–18. doi: 10.1007/BF01318172. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead WE, Wald A, Diamant N, et al. Functional Disorders of the Anorectum. International Working Party Consensus Rome Criteria II. 1999:483–501. [Google Scholar]
- 5.Papachrysostomou M, Smith A. Effects of biofeedback on obstructive defecation – Reconditioning of the defecation reflex? Gut. 1994;35(2):242–45. doi: 10.1136/gut.35.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glia A, Gylin M, Gullberg K, et al. Biofeedback retraining in patients with functional constipation and paradoxical puborectalis contraction: comparison of anal manometry and sphincter electromyography for feedback. Dis Colon Rectum. 1997;40:889–95. doi: 10.1007/BF02051194. [DOI] [PubMed] [Google Scholar]
- 7.Kawimbe BM, Pappachrysostomou M, Binnie NR, et al. Outlet obstruction constipation (anismus) managed by biofeedback. Gut. 1991;32:1175–79. doi: 10.1136/gut.32.10.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martelli H, Devroede G, Arhan P, et al. Mechanisms of idiopathic constipation. Outlet obstruction Gastroenterology. 1978;75:623–31. [PubMed] [Google Scholar]
- 9.Bieijenberg G, Kuipers H. Treatment of spastic pelvic floor syndrome with biofeedback. Dis Colon Rectum. 1987;30:101–11. doi: 10.1007/BF02554946. [DOI] [PubMed] [Google Scholar]
- 10.Thorpe AC, Williams NS, Badenoch DF, et al. Simultaneous dynamic electromyographic portography and cystometrography. Br J Surg. 1993;80(1):115–20. doi: 10.1002/bjs.1800800138. [DOI] [PubMed] [Google Scholar]
- 11.Rao SSC, Tuteja AK, Vellema T, et al. Dyssynergic Defecation: Demographics, Symptoms, Stool Patterns and Quality of Life. J Clin Gastroenterol. 2004;38:680–685. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 12.Bharucha AE, Wald A, Enck P, et al. Functional anorectal disorders. Gastroenterology. 2006;130:1510–8. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 13.Pare P, Ferrazzi S, Thompson D, et al. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Liberman JN, Sandler RS, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol. 1999;94(12):3530–40. doi: 10.1111/j.1572-0241.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 15.Drossman DA, Li Z, Andruzi E, et al. US householder survey of the functional gastrointestinal disorders. Prevalence, sociodemography and health impact. Dig Dis Sci. 1993;38:1569–80. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 16.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–9. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 17.Bradley CS, Kennedy CM, Turcea AM, et al. Constipation in pregnancy: prevalence, symptoms, and risk factors. Obstet Gynecol. 2007;110(6):1351–7. doi: 10.1097/01.AOG.0000295723.94624.b1. [DOI] [PubMed] [Google Scholar]
- 18.Dennison C, Prasad M, Lloyd A, et al. The health related quality of life and economic burden of constipation. Pharmacoeconomics. 2005:23461–76. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 19.Singh G, Lingala V, Wang H, et al. Use of health care resources and cost of care for adults with constipation. Clin Gastroenterol Hepatol. 2007;5(9):1053–8. doi: 10.1016/j.cgh.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Martin BC, Barghout V, Cerulli A. Direct medical costs of constipation in the United States. Manag Care Interface. 2006;19:43–9. [PubMed] [Google Scholar]
- 21.Nehra V, Bruce BK, Rath-Harvey DM, et al. Psychological disorders in patients with evacuation disorders and constipation in a tertiary practice. Am J Gastroenterol. 2000;95:1755–1758. doi: 10.1111/j.1572-0241.2000.02184.x. [DOI] [PubMed] [Google Scholar]
- 22.Rao SS, Seaton K, Miller MJ, et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J Psychosom Res. 2007;63(4):441–9. doi: 10.1016/j.jpsychores.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS, Tuteja AK, Vellema T, et al. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004;38:680–5. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 24.Leroi AM, Berkelmans I, Denis P, et al. Anismus as a Marker of Sexual Abuse. Consequences of Abuse on Anorectal Motility. Dig Dis Sci. 1995;40:1411–6. doi: 10.1007/BF02285184. [DOI] [PubMed] [Google Scholar]
- 25.Ringel Y, Whitehead WE, Tober BB. Sexual and Physical are not Associated with Rectal Hypersensitivity in Patients with Irritable Bowel Syndrome. Gut. 2004;53:838–842. doi: 10.1136/gut.2003.021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvine EJ, Ferrazzi S, Pare P, et al. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–1993. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- 27.Pinho M, Yoshioka K, Keighley MRB. Long term results of anorectal myectomy for chronic constipation. Br J Surg. 1989;76:1163–64. doi: 10.1002/bjs.1800761117. [DOI] [PubMed] [Google Scholar]
- 28.Joo JS, Agachan F, Wolff B, et al. Initial North American experience with botulinum toxin type A for treatment of anismus. Dis Colon Rectum. 1996;39:1107–11. doi: 10.1007/BF02081409. [DOI] [PubMed] [Google Scholar]
- 29.Glia A, Lindberg F, Nilsson LH, et al. Clinical value of symptom assessment in patients with constipation. Dis Colon & Rectum. 1999;42 (11):1401–8. doi: 10.1007/BF02235036. [DOI] [PubMed] [Google Scholar]
- 30.Koch A, Voderholzer WA, Klauser AG, et al. Symptoms in chronic constipation. Dis Colon Rectum. 1998;40(8):902–6. doi: 10.1007/BF02051196. [DOI] [PubMed] [Google Scholar]
- 31.Hull TL, Fazio VW, Schroeder T. Paradoxical puborectalis contraction in patients after pelvic pouch construction. Dis Col Rec. 1995;38 (11):1144–6. doi: 10.1007/BF02048329. [DOI] [PubMed] [Google Scholar]
- 32.Rao SS, Ozturk R, De Ocampo S, et al. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol. 2006;101(3):613–8. doi: 10.1111/j.1572-0241.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 33.Wald A, Chandra R, Chiponis D. Anorectal function and continence mechanisms in childhood encopresis. J Pediatr Gastroenterol Nutr. 1986;5:346–51. doi: 10.1097/00005176-198605000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hobbis JCA, Turpin G, Read NW. Examination of the role of phychosicial factors in the pathogenesis of functional bowel disorders. Gastroenterology. 1995;108:A614. [Google Scholar]
- 35.Chiaioni G, Bassotti G, Monsignori A, et al. Anorectal dysfunction in constipated women with anorexia nervosa. Mayo Clin Proc. 2000;75(10):1015–9. doi: 10.4065/75.10.1015. [DOI] [PubMed] [Google Scholar]
- 36.Kacasick S, Ehrlich SM. Is constipation a disorder of defecation or impaired motility? Am. J Roentgenology. 1996;166(1):63–6. doi: 10.2214/ajr.166.1.8571906. [DOI] [PubMed] [Google Scholar]
- 37.Rao P, Tantiphlachiva K, Attaluri A, Rao SSC. How useful is digital rectal examination in the diagnosis of dyssynergia? AM J Gastroenterol. 2007;102:51. [Google Scholar]
- 38.Lawrentschuk N, Bolton DM. Experience and attitudes of final-year medical students to digital rectal examination. Med J Aust. 2004;181:323–5. doi: 10.5694/j.1326-5377.2004.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 39.Rao SSC, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders. Am J Gastroenterol. 1997;92:469–75. [PubMed] [Google Scholar]
- 40.Rao SSC, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94(3):773–83. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 41.Rao SSC, Azpiroz F, Diamant N. Minimum Standards of Anorectal Manometry. Neurogastroenterol Motil. 2002;14:553–9. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 42.Duthie GS, Bartolo DCC. Anismus. The cause of constipation? Results of investigation and treatment. World Surg. 1992;16:831–35. doi: 10.1007/BF02066978. [DOI] [PubMed] [Google Scholar]
- 43.Rao SSC, Kavlock R, Rao S. Influence of Body Position and Stool Characteristics on Defecation in Humans. Am J Gastroenterol. 2006 doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 44.Pelsang RE, Rao SSC, Welcher K. FECOM: A new artifical stool for assessing defecation. Am J Gastroenterol. 1999;94:183–86. doi: 10.1111/j.1572-0241.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- 45.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116(3):735–60. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 46.Schounten WR, Briel JW, Auwerda JJ, et al. Anismus: fact or fiction? Dis Colon Rectum. 1997;40(9):1033–41. doi: 10.1007/BF02050925. [DOI] [PubMed] [Google Scholar]
- 47.Glia A, Lindberg G, Nilsson LH, et al. Constipation assessed on the basis of colorectal physiology. Scan J Gastroenterol. 1998;33(12):1273–9. doi: 10.1080/00365529850172359. [DOI] [PubMed] [Google Scholar]
- 48.Rao SSC, Mudipalli RS, Stessman M, et al. Investigation of the Utility of Colorectal Function Tests and Rome II Criteria in Dyssynergic Defecation (Anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 49.Stumbo PJ, Hemmingway D, Paulson J, et al. How Useful Is Dietary Management in the Treatment of Chronic Constipation? Supplement to Gastroenterology. 2008;134(4):A-469. [Google Scholar]
- 50.Bassoti G, Gavurri M. Manometeric investigation of high amplitude propagated contractile activity of the human colon. Am J Physiol. 1988:255G660–64. doi: 10.1152/ajpgi.1988.255.5.G660. [DOI] [PubMed] [Google Scholar]
- 51.Rao SSC, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour Colonic Manometry in Slow-transit Constipation. Am J Gastroenterol. 2004;99:2405–16. doi: 10.1111/j.1572-0241.2004.40453.x. [DOI] [PubMed] [Google Scholar]
- 52.Muller-Lissner SA, Kamm MA, Scarpignato C, et al. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232–42. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- 53.Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical Response to Dietary Fiber Treatment of Chronic Constipation. Am J Gastroenterol. 1997;92:95–98. [PubMed] [Google Scholar]
- 54.Brandt LJ, Prather CM, Quigley EM, et al. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol. 2005;100 (Suppl 1):S5–S21. doi: 10.1111/j.1572-0241.2005.50613_2.x. [DOI] [PubMed] [Google Scholar]
- 55.Ramkumar D, Rao SSC. Efficacy and Safety of Traditional Medical Therapies for Chronic Constipation: Systematic Review. Am J Gastroenterol. 2005;100:936–71. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 56.Kline C. Non Prescription Drugs USA. 2000 [Google Scholar]
- 57.Dipalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastrenterol. 2000;95(2):341–2. doi: 10.1111/j.1572-0241.2000.01765.x. [DOI] [PubMed] [Google Scholar]
- 58.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–83. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 59.Lipecka J, Bali M, Thomas A, et al. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol. 2002;282:C805–16. doi: 10.1152/ajpcell.00291.2001. [DOI] [PubMed] [Google Scholar]
- 60.Johanson JF, Morton D, Geenen J. Multicenter, 4-week, double-blind, randomized, placebo-controlled trail of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103(1):170–7. doi: 10.1111/j.1572-0241.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 61.Johanson JFPR, Holland PC, Ueno R. Long-Term efficacy of lubiprostone for the treatment of chronic constipation. Gastroenterology. 2006;130:M117. [Google Scholar]
- 62.Verne GN, Eaker EY, David RD. Colchicine is an Effective Treatment for Patients with Severe Idiopathic Constipation. Gastroenterology. 1995;108:A70. [Google Scholar]
- 63.Roarty TP, Weber F, Soykan I, McCallum RW. Misoprostol in the treatment of chronic refractory constipation: results of a long-term open label trial. Aliment Pharmacol Ther. 1997;11:1059–66. doi: 10.1046/j.1365-2036.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 64.Andersen V, Camilleri M, Busciglio IA, et al. Effects of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterolgoy. 2007;133(3):761–8. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 65.Rao SSC, Welcher K, Pelsang RE. Effects of biofeedback therapy on anorectal function in obstructive defecation. Dig Dis Sci. 1997;42:2197–2205. doi: 10.1023/a:1018846113210. [DOI] [PubMed] [Google Scholar]
- 66.Rao SSC. The Technical aspects of biofeedback therapy for defecation disorders. The Gastroenterologist. 1998;6:96–103. [PubMed] [Google Scholar]
- 67.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–8. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 69.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trail shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-typr constipation. Dis Colon Rectum. 2007;50(4):428–41. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 70.Biofeedback treatment of constipation: a critical review. Dis Colon Rectum. 2003;46(9):1208–17. doi: 10.1007/s10350-004-6717-8. [DOI] [PubMed] [Google Scholar]
- 71.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–64. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 72.Koutsomanis D, Lennard-Jones J, Kamm MA. Prospective study of biofeedback treatment for patients with slow and normal transit constipation. Eur J Gastroenterol Hepatol. 1994;6:131–37. [Google Scholar]
- 73.Patankar SK, Ferrara A, Levy JR, et al. Biofeedback in colorectal practice: a multicenter, statewide, three-year experience. Dis Colon Rectum. 1997;40:827–31. doi: 10.1007/BF02055441. [DOI] [PubMed] [Google Scholar]
- 74.Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with Botulinum A toxin. Lancet. 1988;ii:714–17. doi: 10.1016/s0140-6736(88)90188-2. [DOI] [PubMed] [Google Scholar]
- 75.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]