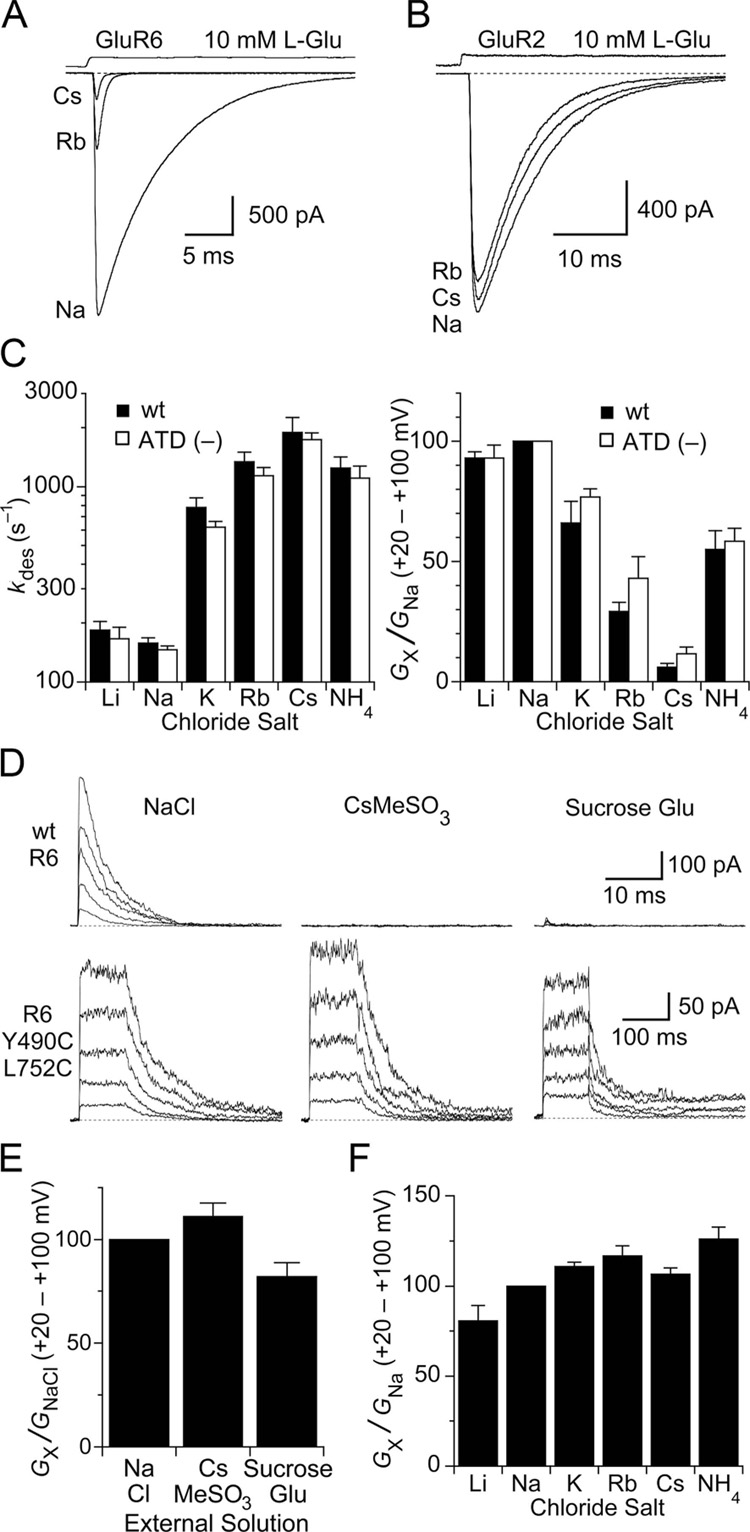
Figure 1. Cations act via sites in the ligand binding domain.
(A) GluR6 responses to 10 mM glutamate are reduced in amplitude and desensitize faster when extracellular Na+ is replaced with Rb+ or Cs+. The effects were fully and rapidly reversible on returning to Na+.
(B) GluR2flip subtype AMPA receptors show little change in glutamate activated responses after exchange of extracellular Na+ by Rb+ or Cs+.
(C) Bar plots summarizing the effects of external monovalent cations on the rate of onset of desensitization (left) and the slope conductance from +20 to +100 mV (right) for responses recorded from wildtype GluR6 and the (ATD–) deletion construct. In all panels, error bars indicate SEM.
(D) Responses to 10 mM glutamate recorded at holding potentials of + 20 to + 100 mV for wild type GluR6 (top row) and the non desensitizing GluR6 Y490C/L752C mutant (bottom row) with NaCl, CsMeSO3 or sucrose in the external solution.
(E) Bar plot of slope conductance measured from + 20 to + 100 mV for the GluR6 Y490C/L752C mutant in NaCl, CsMeSO3 and sucrose solutions.
(F) Bar plot of slope conductance measured from + 20 to + 100 mV for the GluR6 Y490C/L752C mutant in solutions containing Li+, Na+, K+, Rb+, Cs+ or NH4+ as the extracellular monovalent cation.