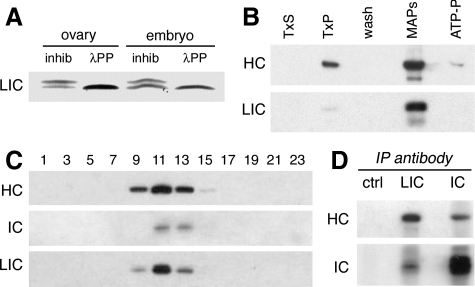
Figure 2.
The LIC is phosphorylated and copurifies with other dynein subunits. (A) An immunoblot of wild-type ovary and embryo extracts probed with the anti-LIC mAb P5F5 identifies a protein doublet with mobility of ∼55/57 kDa. Treatment with λ protein phosphatase (λPP) reduces the doublet to a single band. Phosphatase inhibitors (inhib) were included in the control reaction. Identical results are obtained when the starting material is partially purified dynein. This blot was developed using a chromogenic substrate, to facilitate resolution of the protein bands. (B) Preparation of microtubule-associated proteins from embryos. Like other dynein subunits, the LIC is enriched in the microtubule pellet (TxP) and is released from microtubules in the presence of ATP. Equal total protein is loaded in each lane. TxS and TxP: supernatant and pellet after stabilization of microtubules by Taxol. MAPs and ATP-P, supernatant and pellet after elution with ATP. The blot was probed with antibodies against dynein HC and LIC. The doublet of LIC isoforms is not resolved by the 7.5% PAGE gel, and in this blot the lower band recognized by the anti-LIC antibody is likely to be a proteolytic fragment. (C) Sucrose density gradient fractionation of ovary extracts reveals the cosedimentation of LIC with the dynein HC and dynein IC in a peak corresponding to ∼19S. Equal volumes of alternate fractions are loaded in each lane. (D) Antibodies against the LIC coimmunoprecipitate both the dynein HC and dynein IC subunits from ovary lysate. No dynein is seen in the beads/no antibody control (ctrl).