Figure 4.
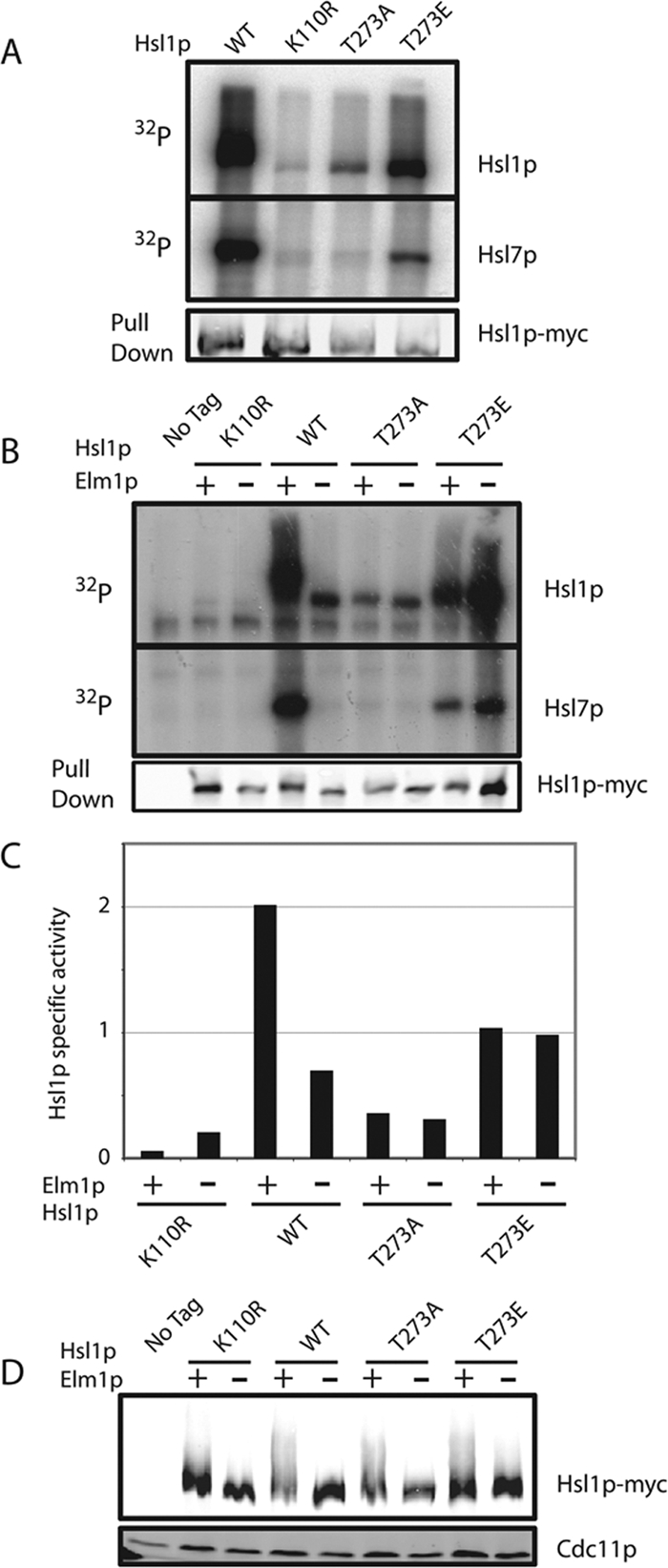
Hsl1p is less active when Elm1p is absent or Hsl1p T273 is not phosphorylatable. (A) Autophosphorylation and Hsl7p phosphorylation is reduced upon mutation of Hsl1p T273. Untagged (DLY1), HSL1-myc (DLY8113), hsl1T273Amyc (DLY7841), HSL1T273Emyc (DLY7451), and hsl1K110Rmyc (DLY8117) cells were grown to exponential phase at 30°C, harvested, and lysed. Hsl1p was immunoprecipitated and incubated with γ-32P-ATP and GST-Hsl7p for 30 min at 30°C before SDS-PAGE and autoradiography. Although the Hsl1p and Hsl7p bands are from the same gel, different exposures are shown. Bottom, a Western blot of the Hsl1p proteins used for the kinase assay. (B) Hsl1p kinase activity depends on Elm1p, but the activity of T273 nonphosphorylatable mutants does not. The same strains as in A together with HSL1-myc elm1Δ (DLY9806), hsl1T273Amyc elm1Δ (DLY9820), HSL1T273Emyc elm1Δ (DLY9804), and hsl1K110Rmyc elm1Δ (DLY9803) were grown and processed as above. Note that the last lane is overloaded. (C) Quantitation of the kinase assay in B. 32P incorporation into Hsl1p in each lane was quantitated by phosphorimager and divided by the amount of Hsl1p in the relevant immunoprecipitate, quantitated using Li-Cor Odyssey software. The ratio is plotted in arbitrary units. (D) The phosphomimic mutation T273E partially restores the Hsl1p mobility shift in elm1Δ cells. The same strains as in B were lysed by TCA precipitation, and Hsl1p-myc and Cdc11p were detected by Western blotting.
