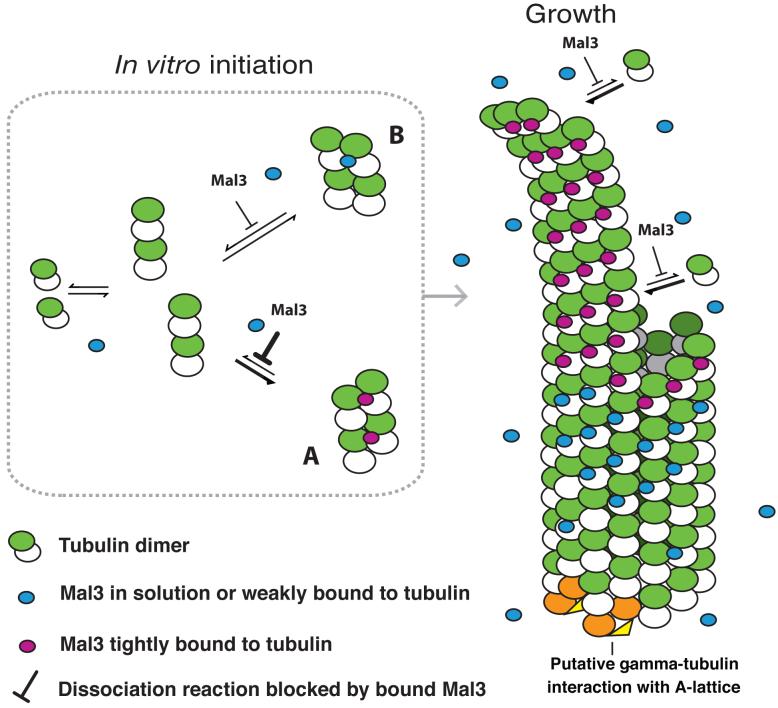
Figure 5.
Model of Mal3 promotion of A-lattice assembly. In vitro tubulin heterodimers will form oligomers that may either continue to assemble or may dissociate again. We propose that Mal3 binds to and stabilizes A-oligomers better than B-oligomers, thus promoting their growth into larger complexes and seeding a future microtubule with a high proportion of A-lattice contacts. As a microtubule grows, Mal3 binds tightly to the tip, which might correspond to an extension with a similar structure to the oligomer seeds. Mal3 binds only weakly to the closed microtubule lattice. White subunits, α-tubulin; green subunits, β-tubulin. Stably bound Mal3 monomers are shown in magenta. More weakly bound Mal3 is shown in blue. γ-tubulin complexes (orange and yellow)27,28 may initiate pure A-lattice assembly in vivo by interacting with both α- and β-tubulin29.